PHYSICAL SCIENCE Introduction Physical Science CHEMISTRY Study of







- Slides: 17

PHYSICAL SCIENCE Introduction

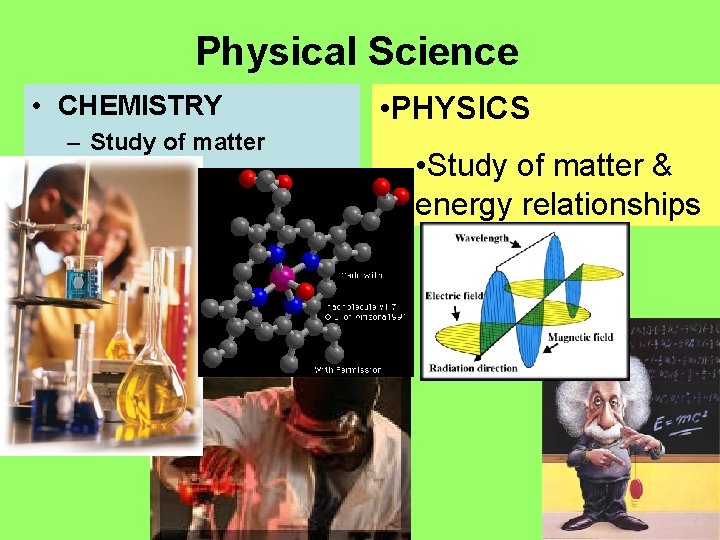
Physical Science • CHEMISTRY – Study of matter • PHYSICS • Study of matter & energy relationships

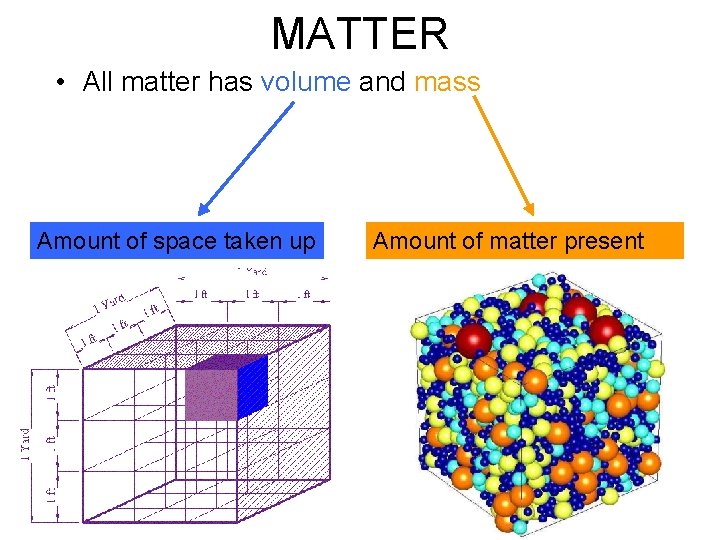
MATTER • All matter has volume and mass Amount of space taken up Amount of matter present

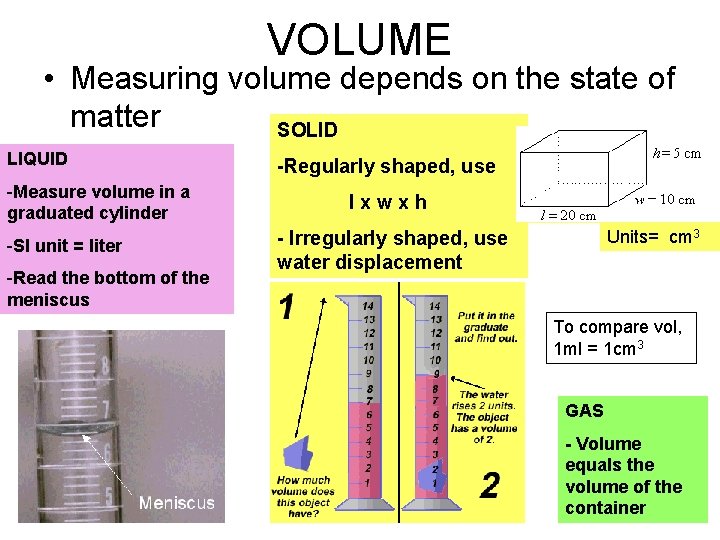
VOLUME • Measuring volume depends on the state of matter SOLID LIQUID -Measure volume in a graduated cylinder -SI unit = liter -Read the bottom of the meniscus -Regularly shaped, use lxwxh Units= cm 3 - Irregularly shaped, use water displacement To compare vol, 1 ml = 1 cm 3 GAS - Volume equals the volume of the container

VOLUME • Which is the best device to measure the volume of a liquid? Why?

VOLUME • Practice reading graduated cylinders – The marks, or calibrations, are different numbers on different graduated cylinders • http: //jchemed. chem. wisc. edu/JCEsoft/Progra ms/Video. CD/CPL/Sample/Modules/gradcyl/gr ad 10 m. L. htm

MASS • To measure the amount of matter present use a BALANCE – Triple beam vs. digital • Tare button- allows measurement of only the contents of a container • Units on balances are grams – Gram is about equal to the mass of a paper clip • SI unit is a kilogram – 1 kg= 2. 2 lbs
Changes in Mass? • Can an object change mass? How?

WEIGHT • Related to mass, but differs • It’s a measure of the force of gravity on matter • Gravity is a force of attraction between two objects – All objects in the universe have gravitational forces on other objects – The more matter, the higher the force of gravity So the higher the weight

WEIGHT • Measured using a spring scale • Units are Newtons (N) • Would you have the same weight on the Earth as on other planets? Why? • Would you have the same mass on the Earth as on other planets? Why?

Wrap-up section 1 • • • 1. 2. 3. 4. Complete “Lab Measurements” Go over DRW 2 -1 Frayer Model of Physical properties Chemical properties Physical changes Chemical changes

• Discuss frayer models of 1. Physical properties 2. Chemical properties 3. Physical changes 4. Chemical changes Chem/Phy Prop 1. Vol 2. Flammability 3. Malleability 4. Ductile • Do “Mystery Powders” Lab • Introduce DENSITY What do we already know?

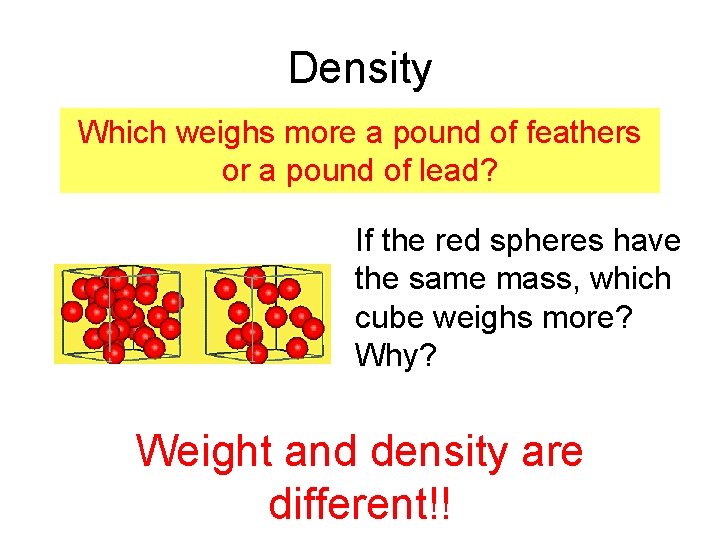
Density Which weighs more a pound of feathers or a pound of lead? If the red spheres have the same mass, which cube weighs more? Why? Weight and density are different!!

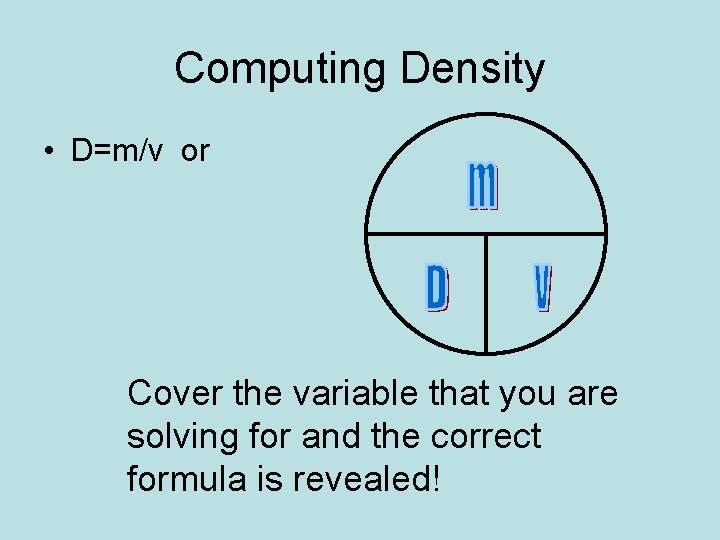
Computing Density • D=m/v or Cover the variable that you are solving for and the correct formula is revealed!

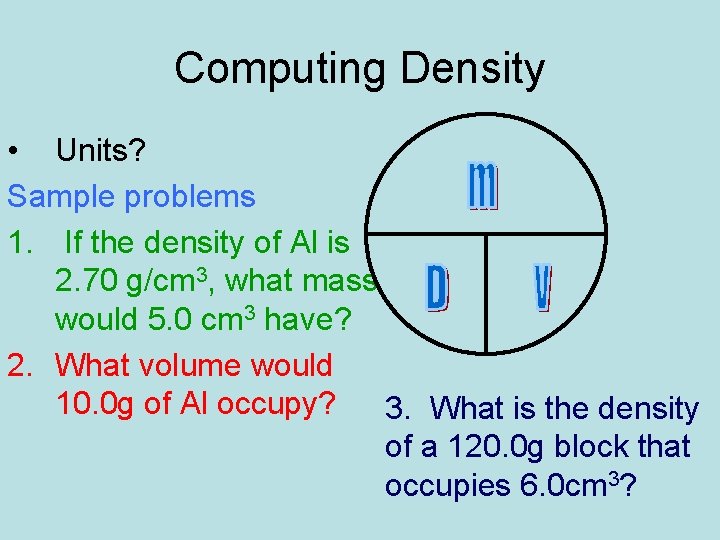
Computing Density • Units? Sample problems 1. If the density of Al is 2. 70 g/cm 3, what mass would 5. 0 cm 3 have? 2. What volume would 10. 0 g of Al occupy? 3. What is the density of a 120. 0 g block that occupies 6. 0 cm 3?

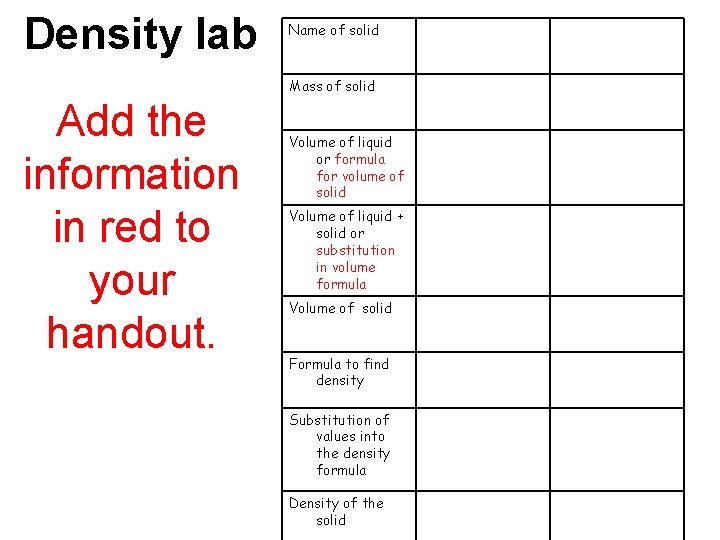
Density lab Name of solid Mass of solid Add the information in red to your handout. Volume of liquid or formula for volume of solid Volume of liquid + solid or substitution in volume formula Volume of solid Formula to find density Substitution of values into the density formula Density of the solid