PHYSICAL SCIENCE EOC REVIEW Nature of Matter Elements





















































- Slides: 63

PHYSICAL SCIENCE EOC REVIEW Nature of Matter Elements Compounds & Mixtures Structure of Matter Chemistry of Elements Chemistry of Compounds Reactions

EOC REVIEW NATURE OF MATTER ® Any substance that has mass and occupies space is called ® A solid ® B matter ® C liquid ® D gas ® ANSWER: B

EOC REVIEW NATURE OF MATTER ® The physical state of matter that has both definite shape and definite volume is ® A solid ® B matter ® C liquid ® D gas ® ANSWER: A

EOC REVIEW NATURE OF MATTER ® The physical state of matter that has a definite volume but no definite shape is ® A solid ® B matter ® C liquid ® D gas ® ANSWER: C

EOC REVIEW NATURE OF MATTER ® The physical state of matter that has no definite volume nor definite shape is ® A solid ® B matter ® C liquid ® D gas ® ANSWER: D

EOC REVIEW NATURE OF MATTER ® The amount of matter in an object is best expressed by its ® A volume ® B density ® C weight ® D mass ® ANSWER: D

EOC REVIEW NATURE OF MATTER ® The force of gravity on an object is called ® A mass ® B volume ® C density ® D weight ® ANSWER: D

EOC REVIEW NATURE OF MATTER ® When a substance changes and still retains its original properties, the change is called ® A chemical ® B exothermic ® C endothermic ® D physical ® ANSWER: D

EOC REVIEW NATURE OF MATTER ® Which of the following is not a necessary characteristic of a chemical change? ® A a change in composition ® B release of energy ® C absorption of energy ® D a change in state ® ANSWER: D

EOC REVIEW COMPOSITION OF MATTER ® Substances that cannot be decomposed to any simpler substances are called ® A elements ® B compounds ® C mixtures ® D none of these ® ANSWER: A

EOC REVIEW COMPOSITION OF MATTER ® Iron, sodium, copper, and silver are examples of ® A metals ® B metalloids ® C nonmetals ® D compounds ® ANSWER: A

EOC REVIEW COMPOSITION OF MATTER ® Metals that reflect light are said to possess the property of ® A malleability ® B ductility ® C conductivity ® D luster ® ANSWER: D

EOC REVIEW COMPOSITION OF MATTER ® Metals that can be hammered into thin sheets are said to possess the property of ® A ductility ® B malleability ® C luster ® D conductivity ® ANSWER: B

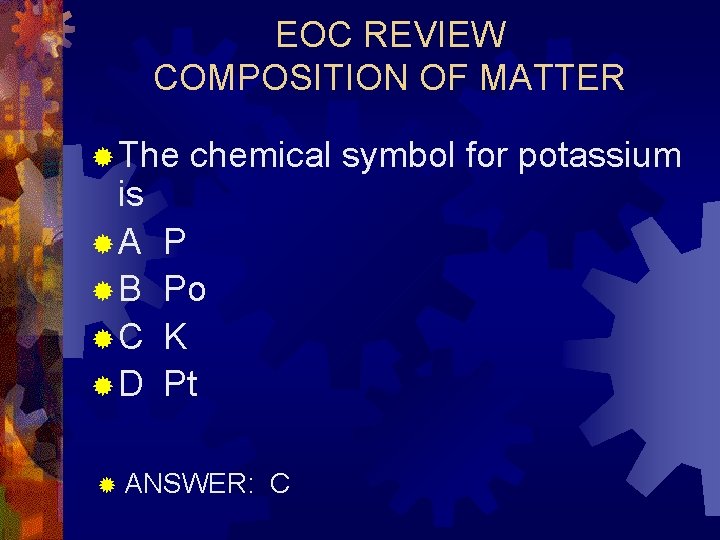
EOC REVIEW COMPOSITION OF MATTER ® The is ®A ®B ®C ®D chemical symbol for potassium P Po K Pt ® ANSWER: C

EOC REVIEW COMPOSITION OF MATTER ® The horizontal rows in the Periodic Table of Elements are known as ® A families ® B groups ® C nonmetals ® D periods ® ANSWER: D

EOC REVIEW COMPOSITION OF MATTER ® The vertical rows in the Periodic Table of Elements are known as ® A metals ® B groups ® C nonmetals ® D periods ® ANSWER: B

EOC REVIEW COMPOSITION OF MATTER ® Compounds consist of two or more elements ® A combined in definite proportions ® B mixed together ® C combined in varying proportions ® D combined physically ® ANSWER: A

EOC REVIEW COMPOSITION OF MATTER ® The number of atoms of oxygen in the formula C 6 H 12 O 6 is ®A 4 ®B 6 ® C 12 ® D 24 ® ANSWER: B

EOC REVIEW STRUCTURE OF MATTER ® The tiny, negatively charged particles first described by JJ Thomson are called ® A nucleus ® B protons ® C electrons ® D neutrons ® ANSWER: C

EOC REVIEW COMPOSITION OF MATTER ® The attraction of a rubbed comb for paper gives evidence that ® A matter is electrical in nature ® B matter is is magnetic ® C matter contains protons ® D matter is electrically neutral ® ANSWER: A

EOC REVIEW COMPOSITION OF MATTER ® When two similarly charged objects are brought close together, they ® A attract each other ® B have no effect on each other ® C stick to each other ® D repel each other ® ANSWER: D

EOC REVIEW COMPOSITION OF MATTER ® An object becomes positively charged when it ® A has an excess of electrons ® B loses electrons ® C has an equal number of protons and neutrons ® D has an equal number of protons and electrons ® ANSWER: B

EOC REVIEW COMPOSITION OF MATTER ® The “plum pudding” model of the atom was proposed by ® A Rutherford ® B Democritus ® C Thomson ® D Bohr ® ANSWER: C

EOC REVIEW COMPOSITION OF MATTER ® The central portion of an atom is called the ® A nucleus ® B shell ® C electron ® D ring ® ANSWER: A

EOC REVIEW COMPOSITION OF MATTER ® In a neutral atom, the number of electrons is ® A equal to the number of neutrons ® B greater than the number of protons ® C less than the number of protons ® D equal to the number of protons ® ANSWER: D

EOC REVIEW COMPOSITION OF MATTER ® Atoms of the same element that differ slightly in mass are called ® A neutrons ® B ions ® C isotopes ® D positrons ® ANSWER: C

EOC REVIEW COMPOSITION OF MATTER ® The differences in the mass of atoms of the same element are attributed to different numbers of ® A protons ® B neutrons ® C electrons ® D positrons ® ANSWER: B

EOC REVIEW COMPOSITION OF MATTER ® The atomic number of an element indicates ® A the number of protons ® B the number of electrons ® C the number of protons and neutrons ® D the number of neutrons ® ANSWER: A

EOC REVIEW COMPOSITION OF MATTER ® The atomic mass of an atom is equal to the mass of the ® A protons ® B protons and neutrons ® C neutrons ® D electrons ® ANSWER: B

EOC REVIEW COMPOSITION OF MATTER ® The maximum number of electrons found in the first level of any atom is ®A 2 ®B 8 ® C 16 ® D 18 ® ANSWER: A

EOC REVIEW COMPOSITION OF MATTER ® In the atom Na, the number of protons is ® A 12 ® B 23 ® C 11 ® D 34 ® ANSWER: C

EOC REVIEW COMPOSITION OF MATTER ® Of the following, the element that has an outermost level that is complete is ® A sodium ® B neon ® C chlorine ® D oxygen ® ANSWER: B

EOC REVIEW COMPOSITION OF MATTER ® To achieve stability when it combines with chlorine, a sodium atom will ® A accept 1 electron ® B accept 7 electrons ® C donate 1 electron ® D donate 7 electrons ® ANSWER: C

EOC REVIEW COMPOSITION OF MATTER ® An element that has fewer than 2 electrons in its first energy level is ® A neon ® B hydrogen ® C oxygen ® D fluorine ® ANSWER: B

EOC REVIEW COMPOSITION OF MATTER symbol X-2 represents an atom a molecule an ion an electron ® The ®A ®B ®C ®D ® ANSWER: C

EOC REVIEW CHEMISTRY OF ELEMENTS ® When ®A ®B ®C ®D metals react chemically, they gain electrons lose electrons gain protons lose protons ® ANSWER: B

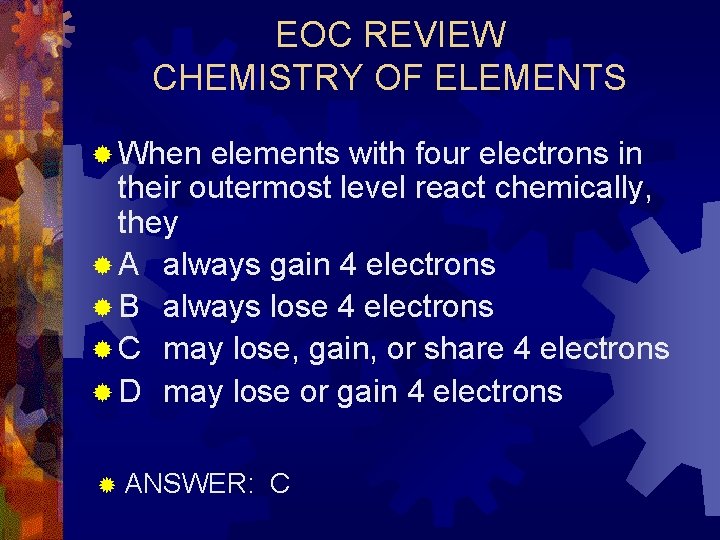
EOC REVIEW CHEMISTRY OF ELEMENTS ® When elements with four electrons in their outermost level react chemically, they ® A always gain 4 electrons ® B always lose 4 electrons ® C may lose, gain, or share 4 electrons ® D may lose or gain 4 electrons ® ANSWER: C

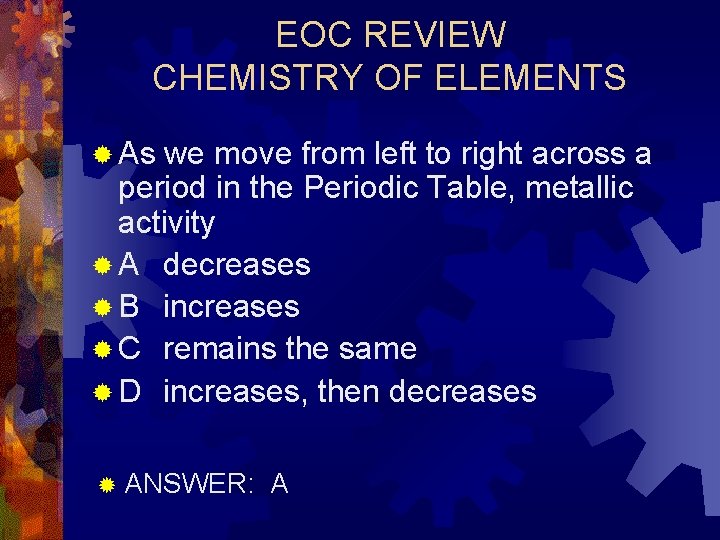
EOC REVIEW CHEMISTRY OF ELEMENTS ® As we move from left to right across a period in the Periodic Table, metallic activity ® A decreases ® B increases ® C remains the same ® D increases, then decreases ® ANSWER: A

EOC REVIEW CHEMISTRY OF ELEMENTS ® In the Periodic Table, the elements that exhibits the strongest nonmetallic properties is found ® A on the upper left side ® B on the lower left side ® C on the upper right side ® D on the lower right side ® ANSWER: C

EOC REVIEW CHEMISTRY OF ELEMENTS ® In the outermost electron level of most metalloids, the number of electrons is ®A 8 ®B 4 ®C 2 ®D 6 ® ANSWER: B

EOC REVIEW CHEMISTRY OF ELEMENTS ® In any group of metals in the Periodic Table, the most active metal in the group is found ® A at the top ® B in the middle ® C at the bottom ® D in different places depending on the group ® ANSWER: C

EOC REVIEW CHEMISTRY OF ELEMENTS ® Potassium is more reactive than sodium and both metals are found in the same group. The atomic radius of potassium is ® A larger than that of sodium ® B the same as that of sodium ® C smaller than that of sodium ® D variable ® ANSWER: A

EOC REVIEW CHEMISTRY OF ELEMENTS ® The most active nonmetal in a group is found ® A at the bottom ® B at the top ® C in the middle ® D in different places depending on the group ® ANSWER: B

EOC REVIEW CHEMISTRY OF ELEMENTS ® The oxidation state of aluminum is +3. This means that, in a chemical reaction, aluminum is most likely to ® A gain 5 electrons ® B gain 3 electrons ® C give away 5 electrons ® D give away 3 electrons ® ANSWER: D

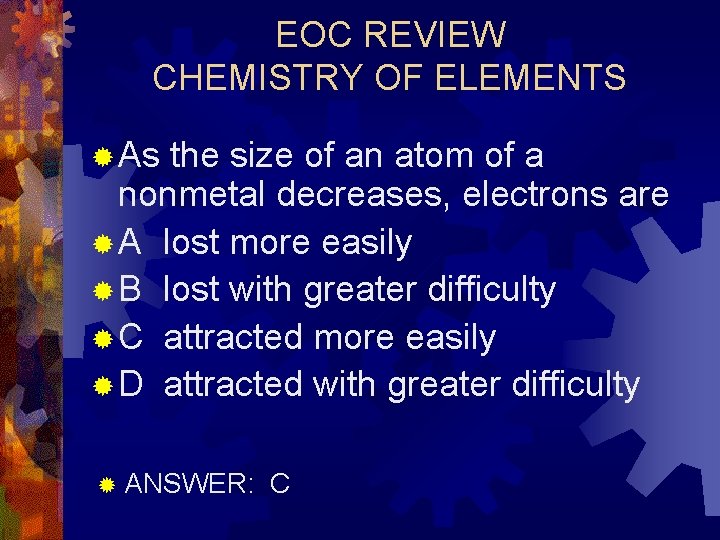
EOC REVIEW CHEMISTRY OF ELEMENTS ® As the size of an atom of a nonmetal decreases, electrons are ® A lost more easily ® B lost with greater difficulty ® C attracted more easily ® D attracted with greater difficulty ® ANSWER: C

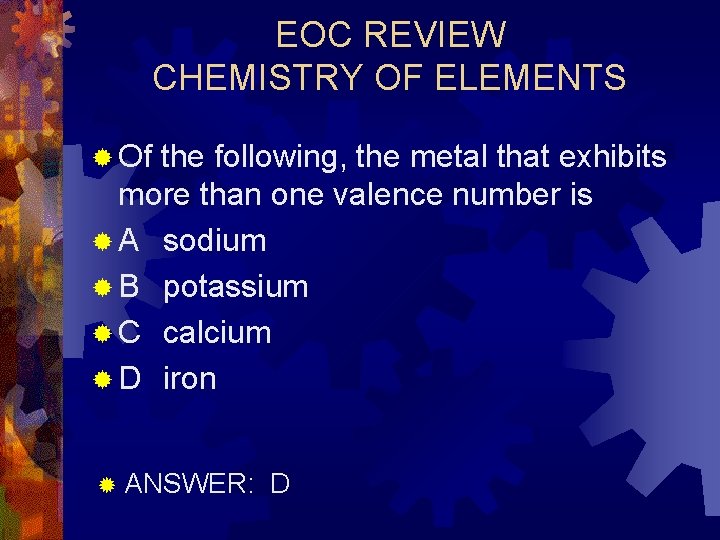
EOC REVIEW CHEMISTRY OF ELEMENTS ® Of the following, the metal that exhibits more than one valence number is ® A sodium ® B potassium ® C calcium ® D iron ® ANSWER: D

EOC REVIEW CHEMISTRY OF ELEMENTS ® Of the following nonmetals, the element that does not exhibit more than one valence electron number is ® A fluorine ® B sulfur ® C carbon ® D nitrogen ® ANSWER: A

EOC REVIEW CHEMISTRY OF ELEMENTS ® In the compound Fe. O, iron has an oxidation number of ® A -2 ® B +2 ® C +3 ® D -3 ® ANSWER: B

EOC REVIEW CHEMISTRY OF COMPOUNDS ® To indicate the number of atoms of an element in a compound, you use ® A a subscript to the right of the symbol ® B an exponent to the right of the symbol ® C a subscript to the left of the symbol ® D an exponent to the left of the symbol ® ANSWER: A

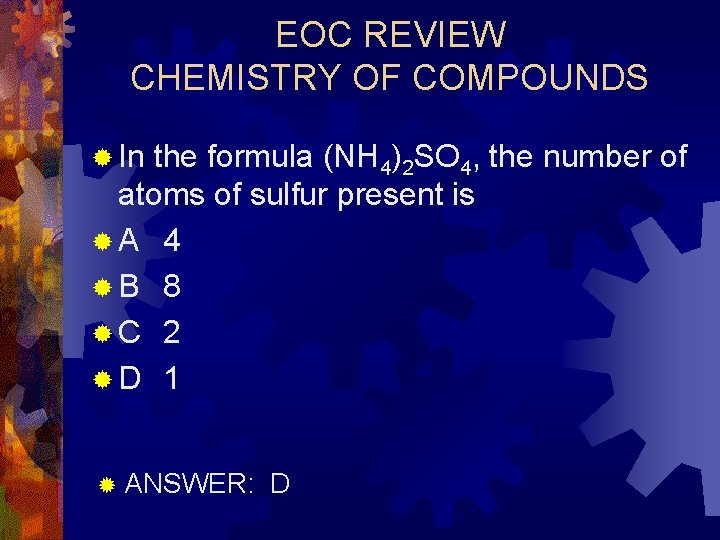
EOC REVIEW CHEMISTRY OF COMPOUNDS ® In the formula (NH 4)2 SO 4, the number of atoms of sulfur present is ®A 4 ®B 8 ®C 2 ®D 1 ® ANSWER: D

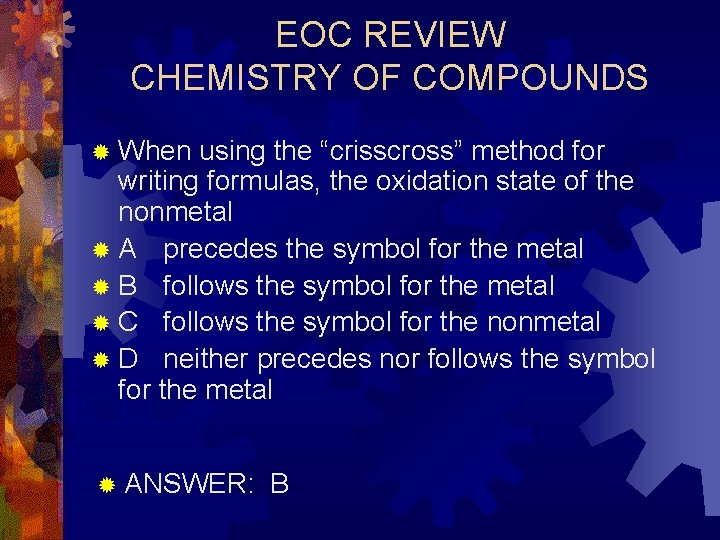
EOC REVIEW CHEMISTRY OF COMPOUNDS ® When using the “crisscross” method for writing formulas, the oxidation state of the nonmetal ® A precedes the symbol for the metal ® B follows the symbol for the metal ® C follows the symbol for the nonmetal ® D neither precedes nor follows the symbol for the metal ® ANSWER: B

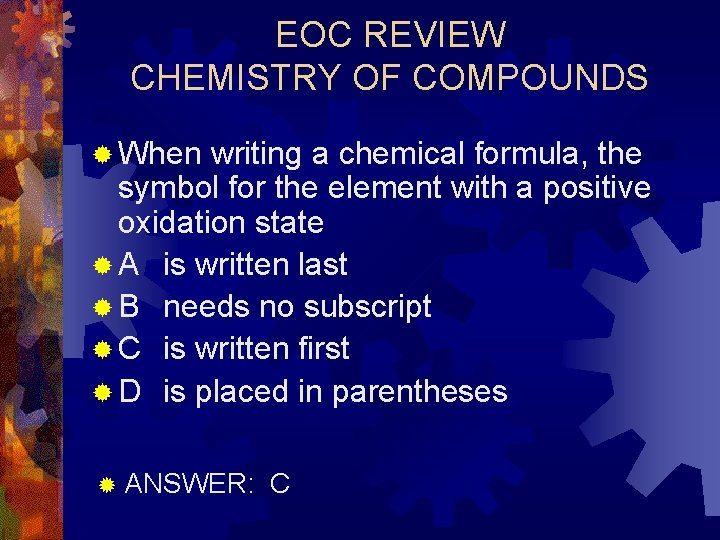
EOC REVIEW CHEMISTRY OF COMPOUNDS ® When writing a chemical formula, the symbol for the element with a positive oxidation state ® A is written last ® B needs no subscript ® C is written first ® D is placed in parentheses ® ANSWER: C

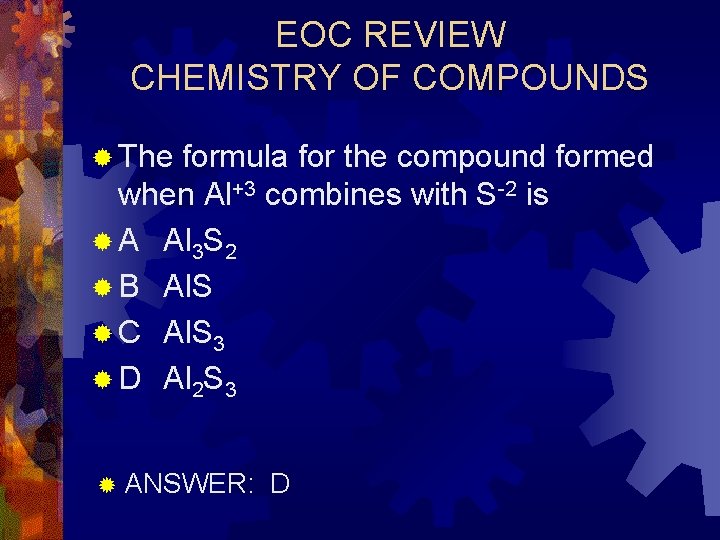
EOC REVIEW CHEMISTRY OF COMPOUNDS ® The formula for the compound formed when Al+3 combines with S-2 is ® A Al 3 S 2 ® B Al. S ® C Al. S 3 ® D Al 2 S 3 ® ANSWER: D

EOC REVIEW CHEMISTRY OF COMPOUNDS ® The compound whose formula is Zn. S is named ® A zinc sulfate ® B zinc sulfite ® C zinc sulfide ® D zinc and sulfur ® ANSWER: C

EOC REVIEW CHEMISTRY OF COMPOUNDS ® The compound whose formula is Ca(OH)2 is named ® A calcium oxygen hydride ® B calcium hydroxide ® C carbon hydrogen oxide ® D calcium oxide ® ANSWER: B

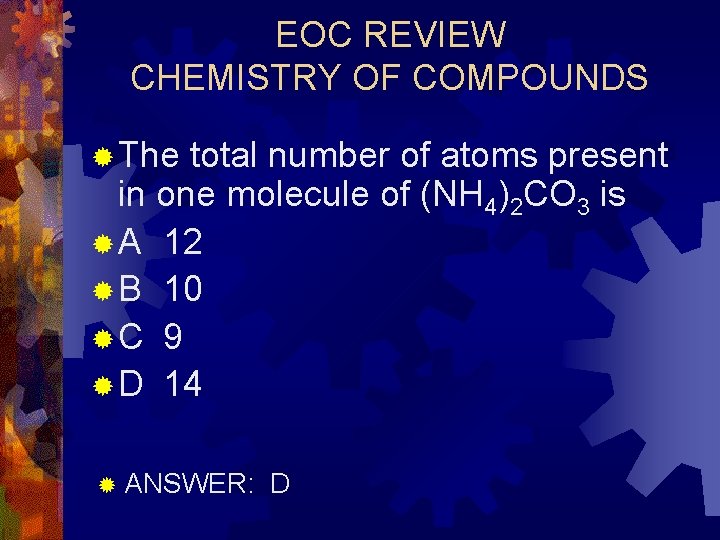
EOC REVIEW CHEMISTRY OF COMPOUNDS ® The total number of atoms present in one molecule of (NH 4)2 CO 3 is ® A 12 ® B 10 ®C 9 ® D 14 ® ANSWER: D

EOC REVIEW CHEMISTRY OF COMPOUNDS ® When an atom of aluminum with a valence of +3 unites to form a compound, it ® A donates 3 electrons ® B accepts 3 electrons ® C donates 5 electrons ® D accepts 5 electrons ® ANSWER: A

EOC REVIEW REACTIONS ®A chemical equation describes ® A only the reactants ® B only the products ® C neither the reactants nor the products ® D both the reactants and the products ® ANSWER: D

EOC REVIEW REACTIONS ® In a chemical reaction, the total mass of the reactants ® A is greater than the total mass of the products ® B is equal to the total mass of the products ® C is less than the total mass of the products ® D cannot be determined ® ANSWER: B

EOC REVIEW REACTIONS ® To satisfy the law of conservation of matter, an equation ® A must first be written in words ® B should show the atomic masses of the reactants and products ® C must be balanced ® D must indicate the physical state of each of the reactants and products ® ANSWER: C

EOC REVIEW REACTIONS ® To ®A ®B ®C ®D balance an equation, you use exponents subscripts superscripts coefficents ® ANSWER: D

EOC REVIEW REACTIONS ® The number of atoms of oxygen present in 2 Al(NO 3)3 is ® A 18 ®B 6 ®C 9 ®D 8 ® ANSWER: A

EOC REVIEW REACTIONS ® In a synthesis reaction, ® A two metals change places ® B two or more elements form a single compound ® C a compound is broken down ® D heat energy is not released ® ANSWER: B