Physical Science EOC Review Marilyn Pendley Instructor CCCMC







- Slides: 36

Physical Science EOC Review Marilyn Pendley Instructor CCCMC

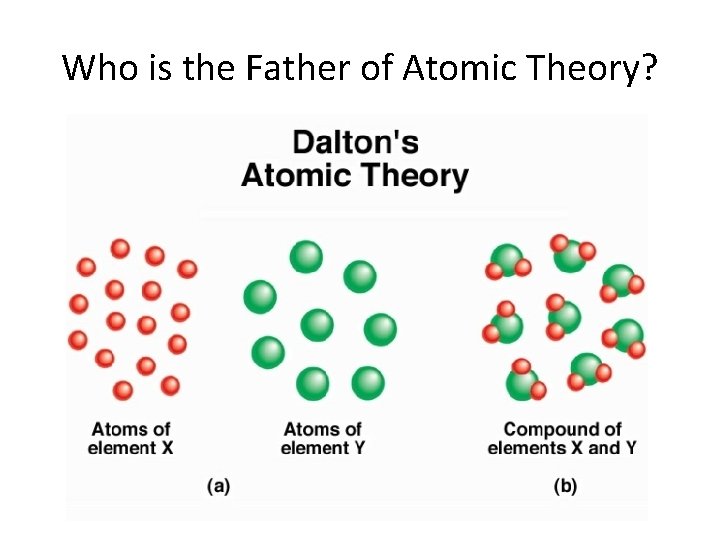
Who is the Father of Atomic Theory?

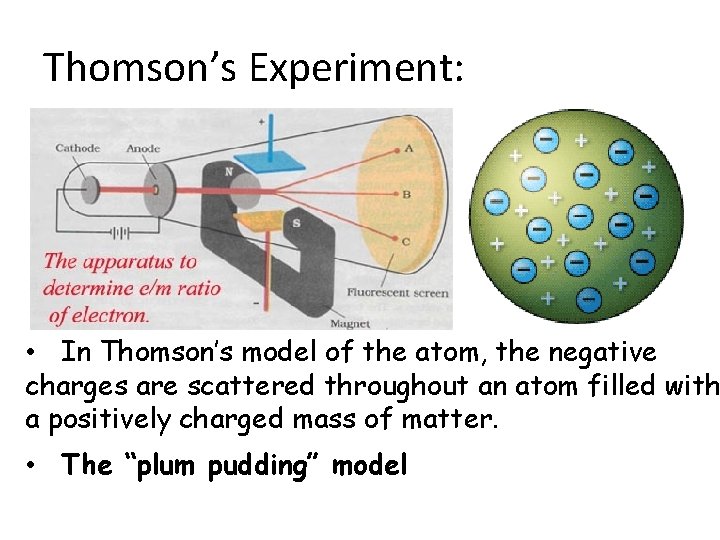
Thomson’s Experiment: • In Thomson’s model of the atom, the negative charges are scattered throughout an atom filled with a positively charged mass of matter. • The “plum pudding” model

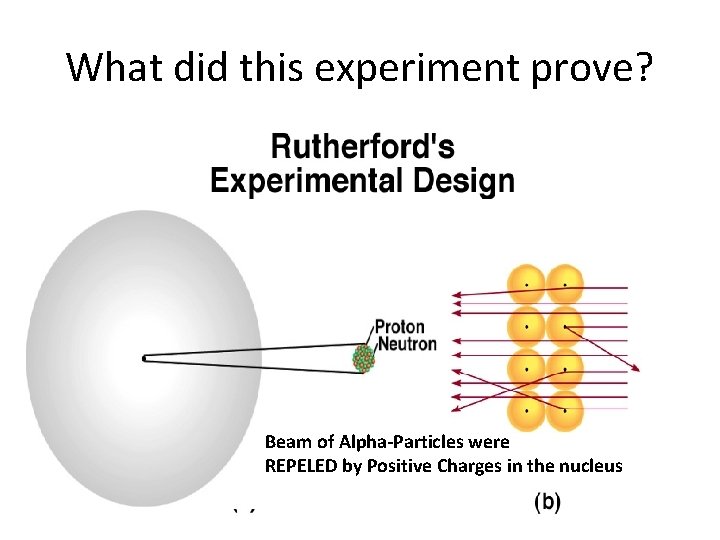
What did this experiment prove? Beam of Alpha-Particles were REPELED by Positive Charges in the nucleus

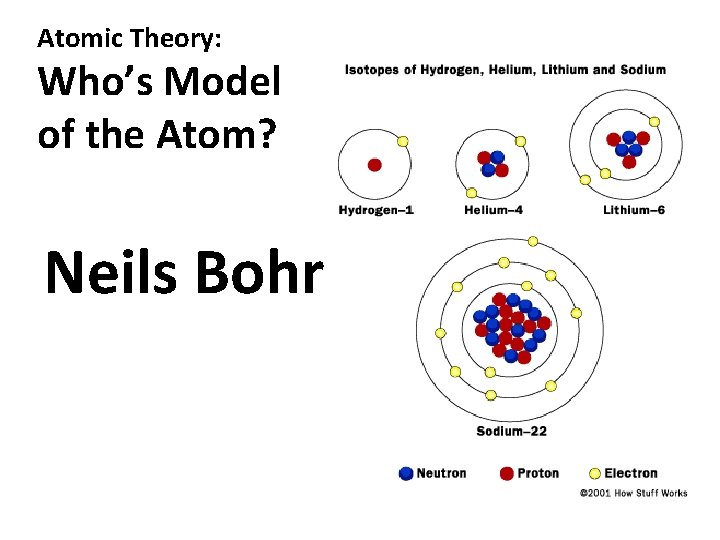
Atomic Theory: Who’s Model of the Atom? Neils Bohr

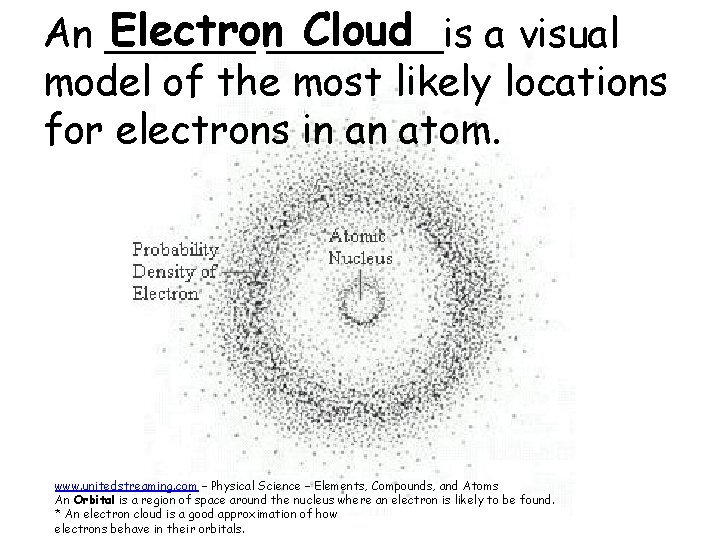
Electron_______is Cloud An ______ a visual model of the most likely locations for electrons in an atom. www. unitedstreaming. com – Physical Science – Elements, Compounds, and Atoms An Orbital is a region of space around the nucleus where an electron is likely to be found. * An electron cloud is a good approximation of how electrons behave in their orbitals.

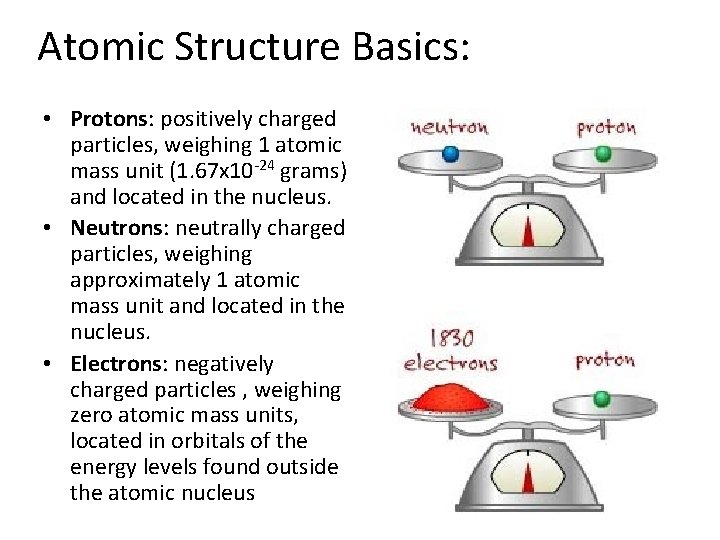
Atomic Structure Basics: • Protons: positively charged particles, weighing 1 atomic mass unit (1. 67 x 10 -24 grams) and located in the nucleus. • Neutrons: neutrally charged particles, weighing approximately 1 atomic mass unit and located in the nucleus. • Electrons: negatively charged particles , weighing zero atomic mass units, located in orbitals of the energy levels found outside the atomic nucleus

Atomic Number: • The number of protons • Play the Name the Atom in an atom determines Game at: what element it is. • http: //www. learner. org • Add or subtract even /interactives/periodic/b one proton from an asics_interactive. html atom of any element and you no longer have the original element in any form. Now you have a different element!

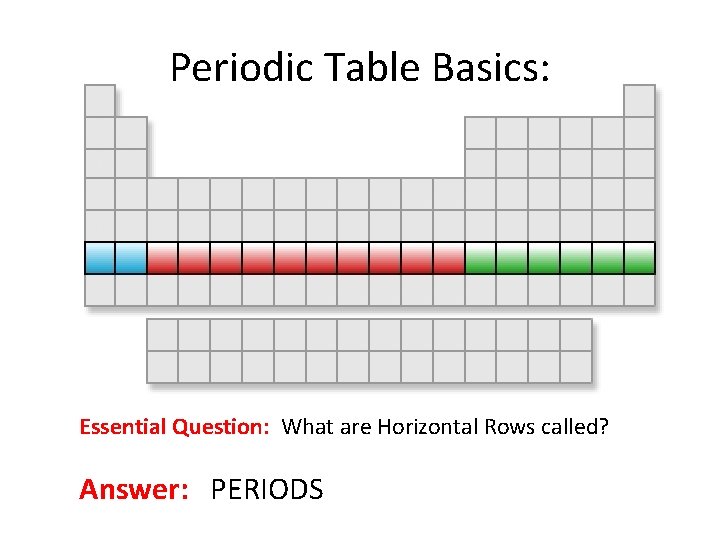
Periodic Table Basics: Essential Question: What are Horizontal Rows called? Answer: PERIODS

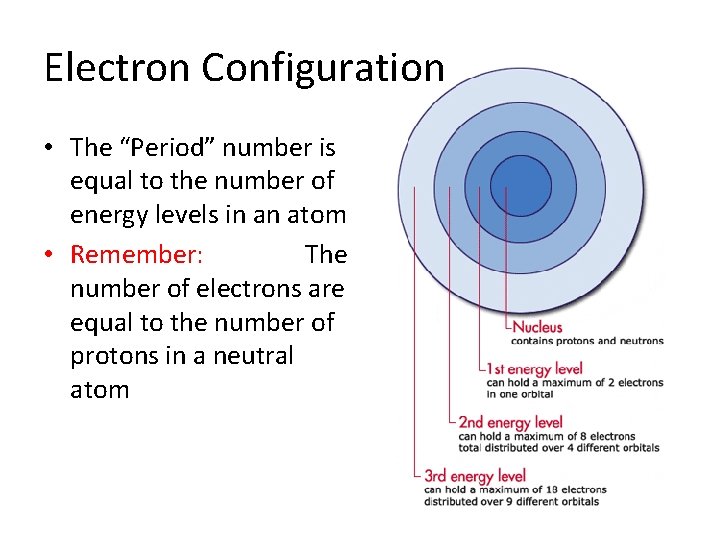
Electron Configuration • The “Period” number is equal to the number of energy levels in an atom • Remember: The number of electrons are equal to the number of protons in a neutral atom

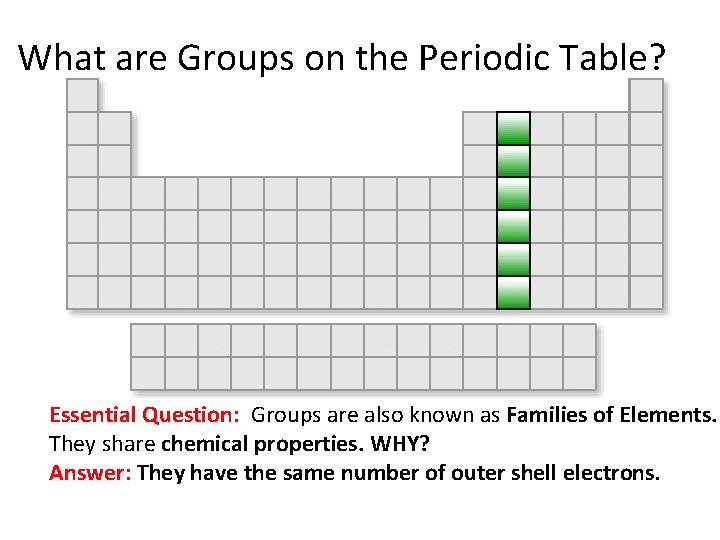
What are Groups on the Periodic Table? Essential Question: Groups are also known as Families of Elements. They share chemical properties. WHY? Answer: They have the same number of outer shell electrons.

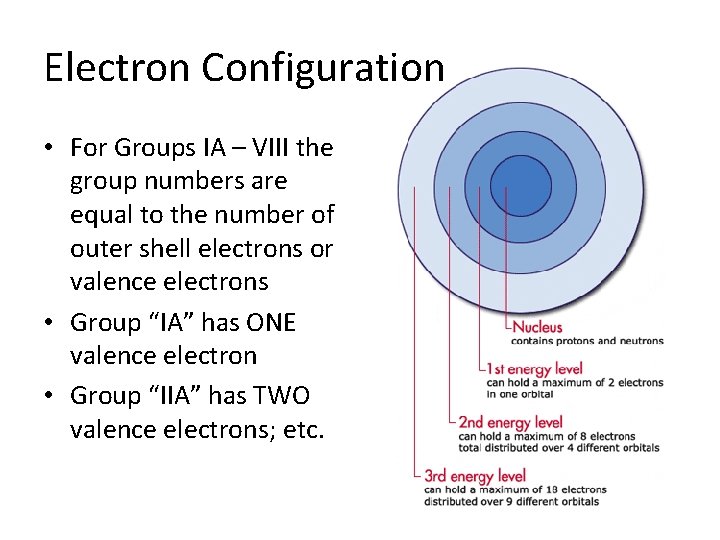
Electron Configuration • For Groups IA – VIII the group numbers are equal to the number of outer shell electrons or valence electrons • Group “IA” has ONE valence electron • Group “IIA” has TWO valence electrons; etc.

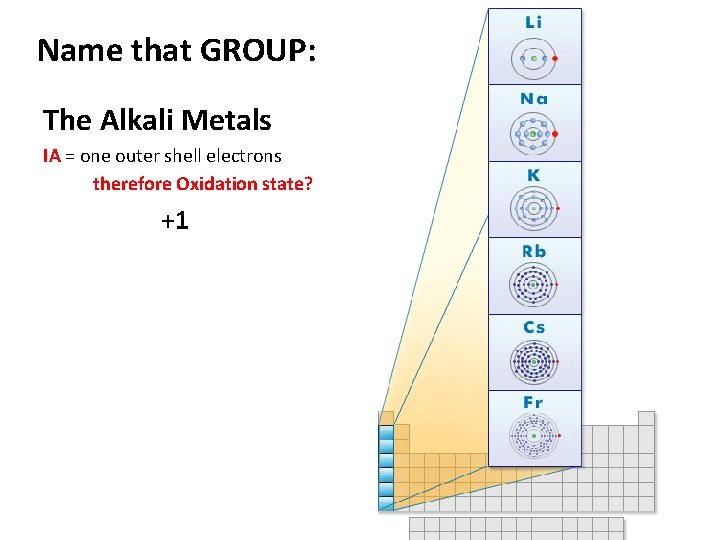
Name that GROUP: The Alkali Metals IA = one outer shell electrons therefore Oxidation state? +1

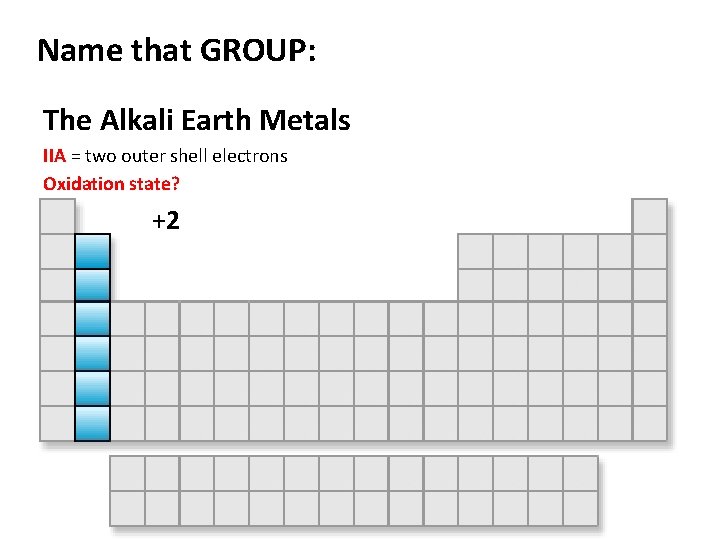
Name that GROUP: The Alkali Earth Metals IIA = two outer shell electrons Oxidation state? +2

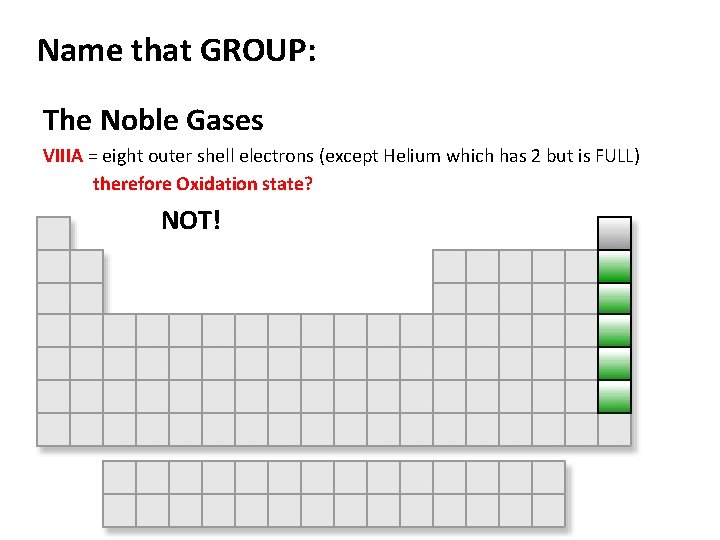
Name that GROUP: The Noble Gases VIIIA = eight outer shell electrons (except Helium which has 2 but is FULL) therefore Oxidation state? NOT!

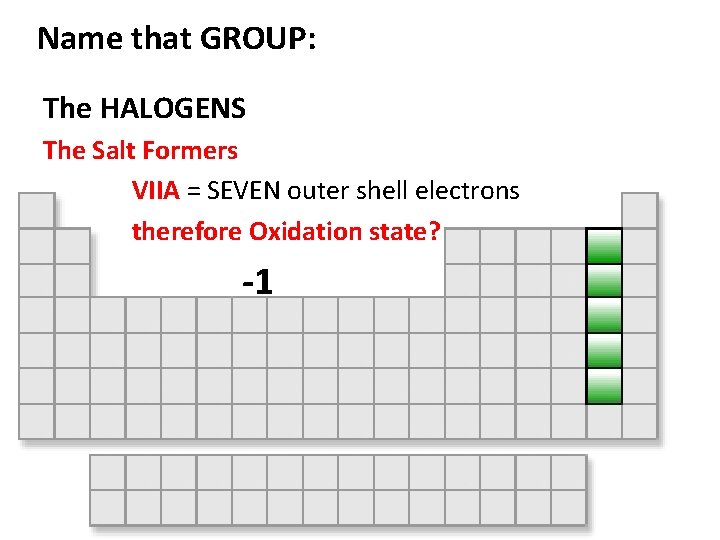
Name that GROUP: The HALOGENS The Salt Formers VIIA = SEVEN outer shell electrons therefore Oxidation state? -1

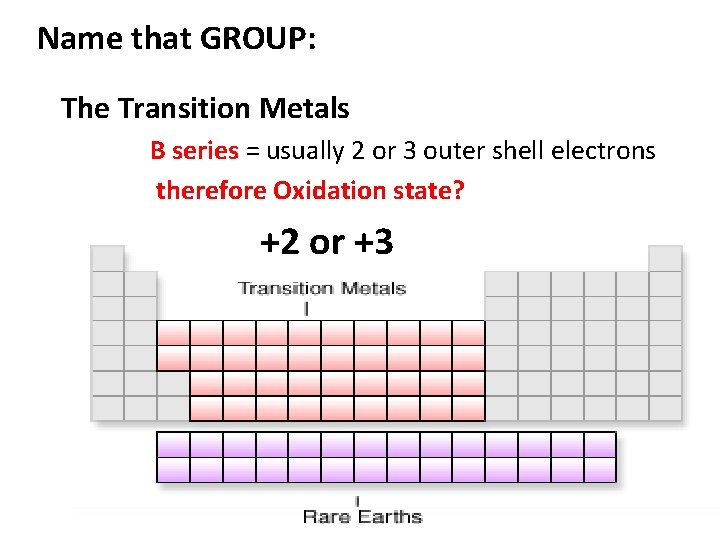
Name that GROUP: The Transition Metals B series = usually 2 or 3 outer shell electrons therefore Oxidation state? +2 or +3

Chemical Bonding: • Three types of bonding Game: Ionic Bonding • Ionic • Covalent • Metallic Bonding Animations and short clips AWESOME MOVIE ON BONDING 30 minutes long

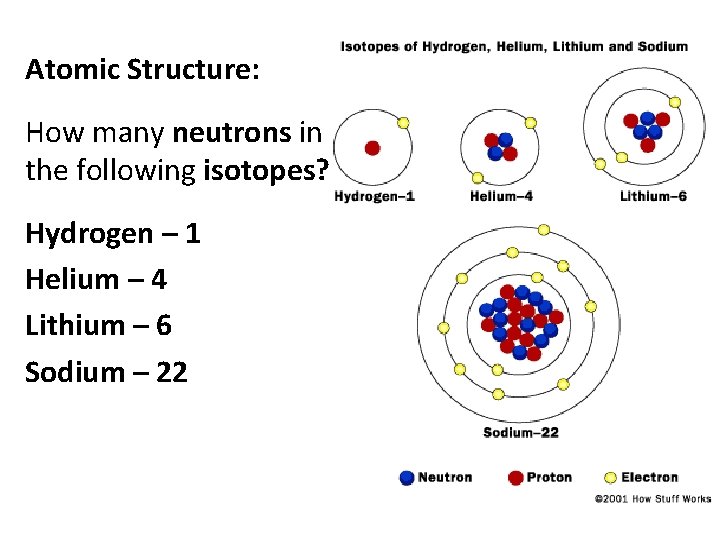
Atomic Structure: How many neutrons in the following isotopes? Hydrogen – 1 Helium – 4 Lithium – 6 Sodium – 22

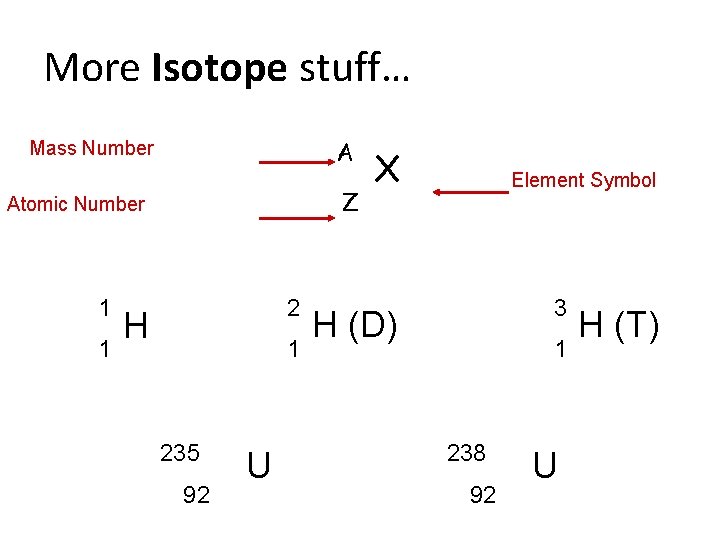
More Isotope stuff… A Mass Number Z Atomic Number 1 1 2 H 1 235 92 U X Element Symbol 3 H (D) 1 238 92 U H (T)

Nuclear Energy Essential Questions: • Why is it worth the RISK? ? • Tremendous OUTPUT of ENERGY!! • Fission or Fusion? • Fission! Atoms of U-235 are split • Use the link to see how Nuclear Fusion works: http: //science. howstuffworks. com/fusion-reactor. htm/printable

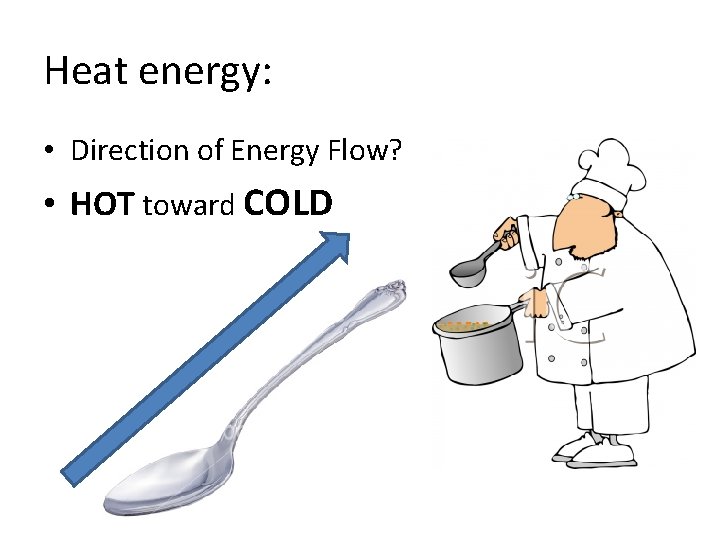
Heat energy: • Direction of Energy Flow? • HOT toward COLD

States of Matter • Watch what happens to molecules when heated by doing cool virtual experiments. • Click on the icon below to get started:

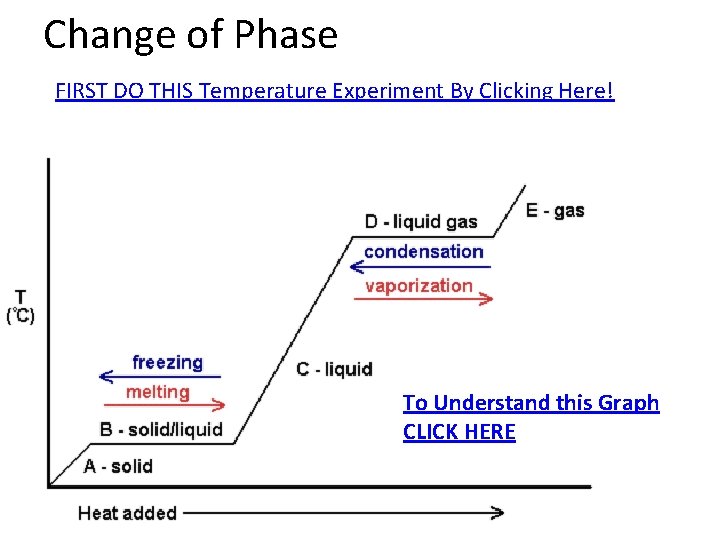
Change of Phase FIRST DO THIS Temperature Experiment By Clicking Here! To Understand this Graph CLICK HERE

Chemical or Physical Change? Chemical Change • New substances formed with new properties • Examples: • Rusting • Gas forming during a reaction (bubbles) • A precipitant forming during a reaction Physical Change • No new substances formed • Examples: • Ice melting • Water evaporating • Dry ice subliming into Carbon dioxide • Salt or sugar dissolving in water

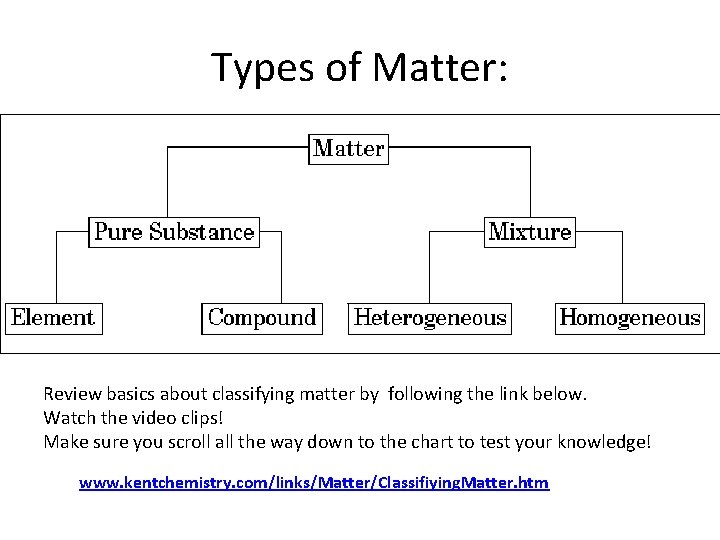
Types of Matter: Review basics about classifying matter by following the link below. Watch the video clips! Make sure you scroll all the way down to the chart to test your knowledge! www. kentchemistry. com/links/Matter/Classifiying. Matter. htm

Where is Magnetism Concentrated? Magnetism is strongest at the POLES

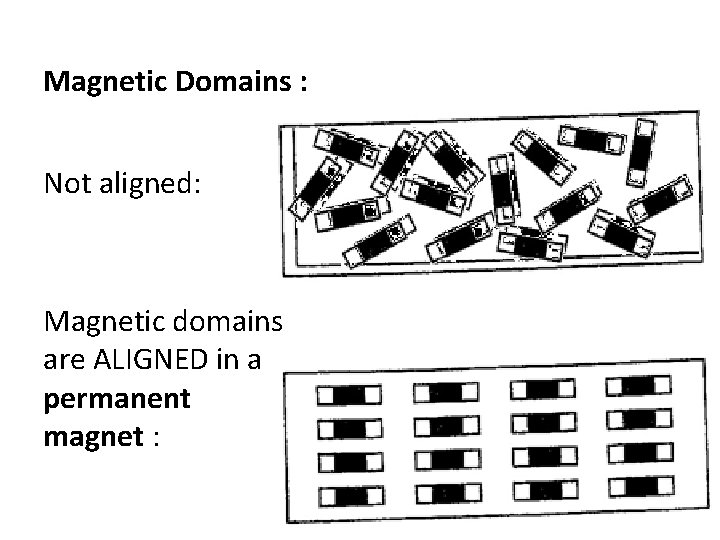
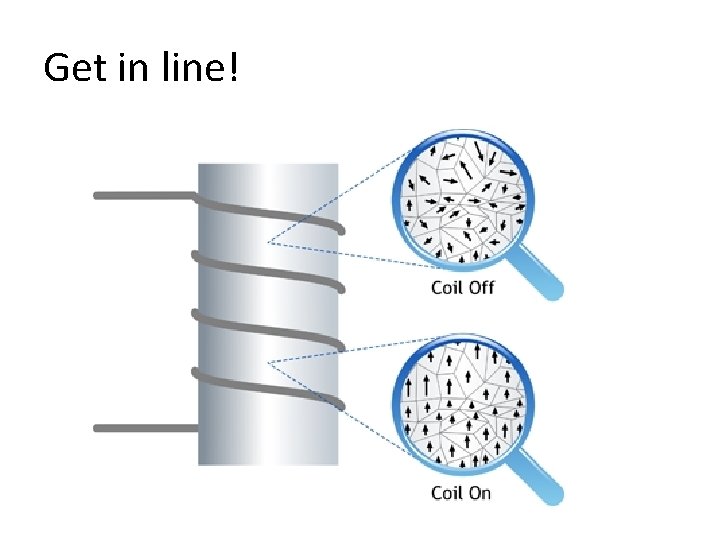
Magnetic Domains : Not aligned: Magnetic domains are ALIGNED in a permanent magnet :

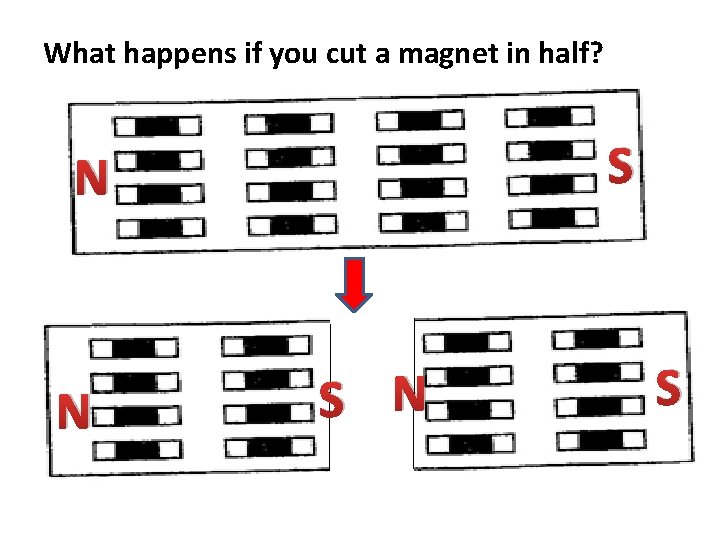
What happens if you cut a magnet in half? S N N S

Electricity and Magnetism:

Get in line!

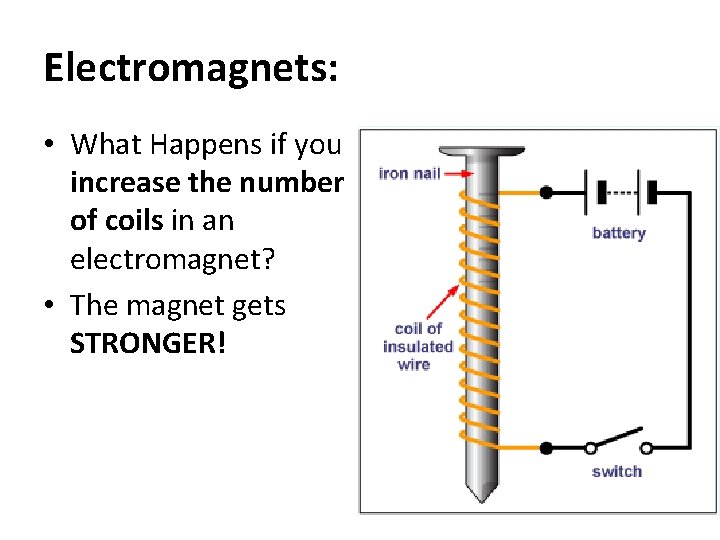
Electromagnets: • What Happens if you increase the number of coils in an electromagnet? • The magnet gets STRONGER!

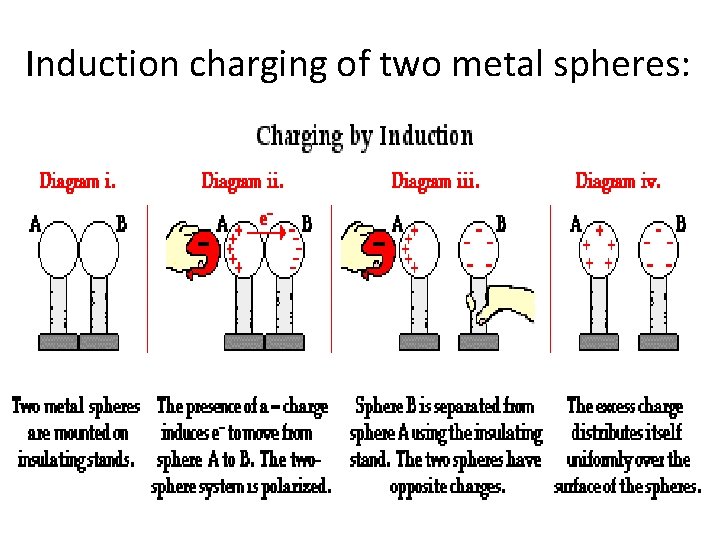
Induction charging of two metal spheres:


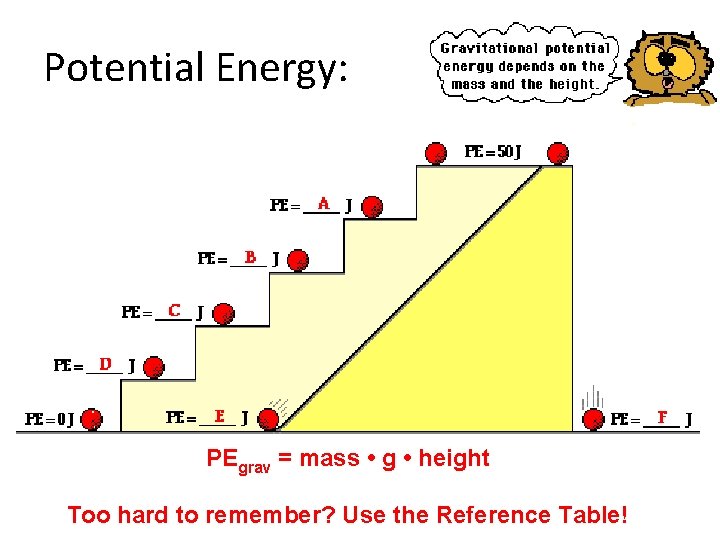
Potential Energy: PEgrav = mass • g • height Too hard to remember? Use the Reference Table!

Answer key: A: PE = 40 J (since the same mass is elevated to 4/5 -ths height of the top stair) B: PE = 30 J (since the same mass is elevated to 3/5 -ths height of the top stair) C: PE = 20 J (since the same mass is elevated to 2/5 -ths height of the top stair) D: PE = 10 J (since the same mass is elevated to 1/5 -ths height of the top stair) E and F: PE = 0 J (since the same mass is at the same zero height position as shown for the bottom stair Note since PEgrav = m * • g • h Doubling of the height will result in a doubling of the gravitational potential energy. • A tripling of the height will result in a tripling of the gravitational potential energy.