PHYSICAL SCIENCE Chapter 1 The Nature of Science




















- Slides: 43

PHYSICAL SCIENCE Chapter 1: The Nature of Science Section 1: The Methods of Science 1

What is science? § The term science is derived from the latin word scientia, meaning “knowledge. ” 2

There are 3 Major Categories of Science § 1. Earth science— 7 th grade—investigates Earth and space § 2. Life science— 8 th grade—deals with living things § 3. Physical science — 9 th grade—study of matter and energy 3

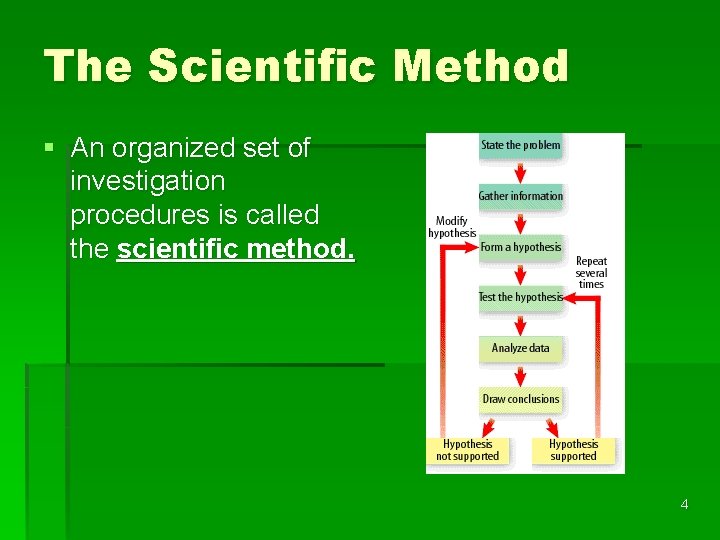
The Scientific Method § An organized set of investigation procedures is called the scientific method. 4

STEP 1 § STATE THE PROBLEM (after making observations) § The problem is often stated in the form of a question (Why…? How…? ) 5

STEP 2 § RESEARCH AND GATHER INFORMATION § Learn about the background of the problem. § What other tests have scientists already performed? 6

STEP 3 § FORM A HYPOTHESIS—A hypothesis is a possible explanation for a problem. § “Educated Guess” § Prediction 7

STEP 4 § TESTING A HYPOTHESIS § Make observations § Build a model § Perform an experiment 8

STEP 5 § ANALYZE THE DATA—Record observations into easy-to-read tables and graphs. § Include all results, even unexpected ones. (NO BIAS) 9

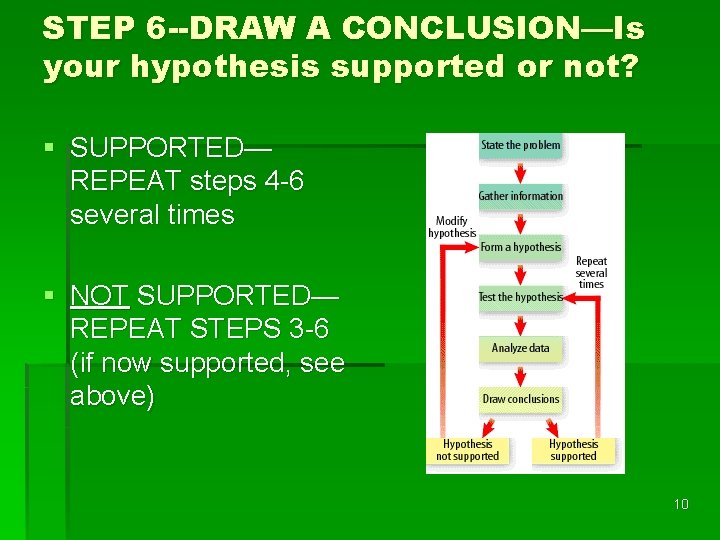
STEP 6 --DRAW A CONCLUSION—Is your hypothesis supported or not? § SUPPORTED— REPEAT steps 4 -6 several times § NOT SUPPORTED— REPEAT STEPS 3 -6 (if now supported, see above) 10

VARIABLES § A variable is a quantity that can have more than a single value. § An experiment usually contains at least 2 variables. 11

EXPERIMENT § Which brand of fertilizer helps plants to grow the biggest? 12

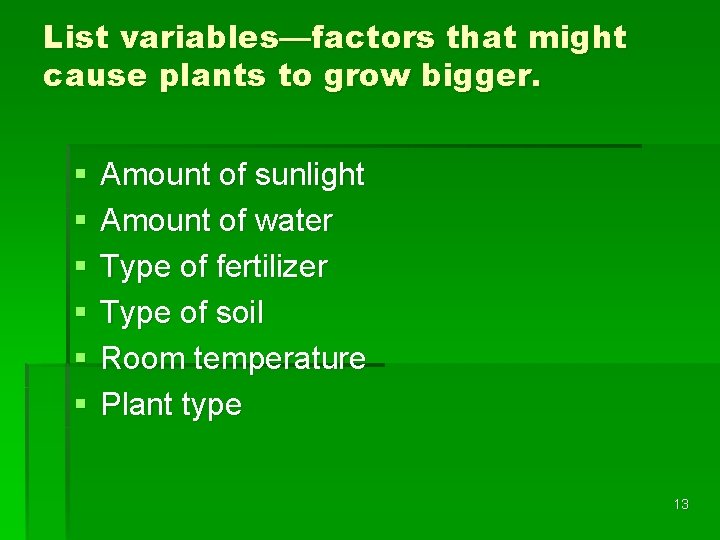
List variables—factors that might cause plants to grow bigger. § § § Amount of sunlight Amount of water Type of fertilizer Type of soil Room temperature Plant type 13

What is the independent variable? § The variable you change to see how it will affect the dependent variable. § The scientist is able to choose the independent variable. § Ex. The brand of fertilizer 14

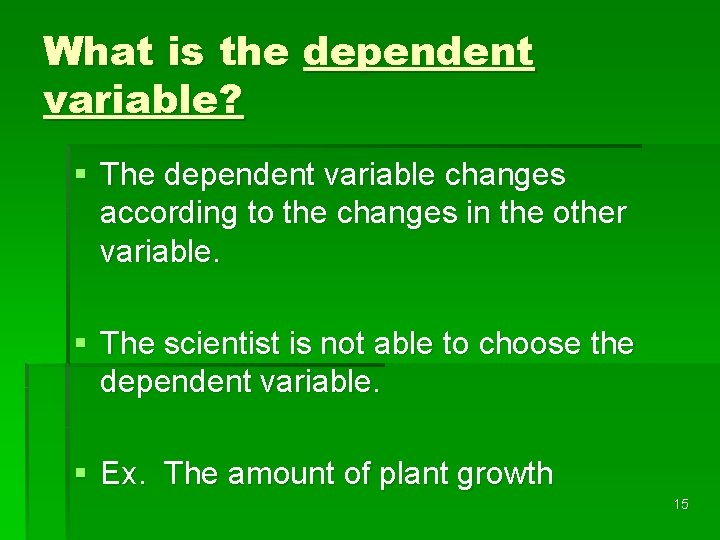
What is the dependent variable? § The dependent variable changes according to the changes in the other variable. § The scientist is not able to choose the dependent variable. § Ex. The amount of plant growth 15

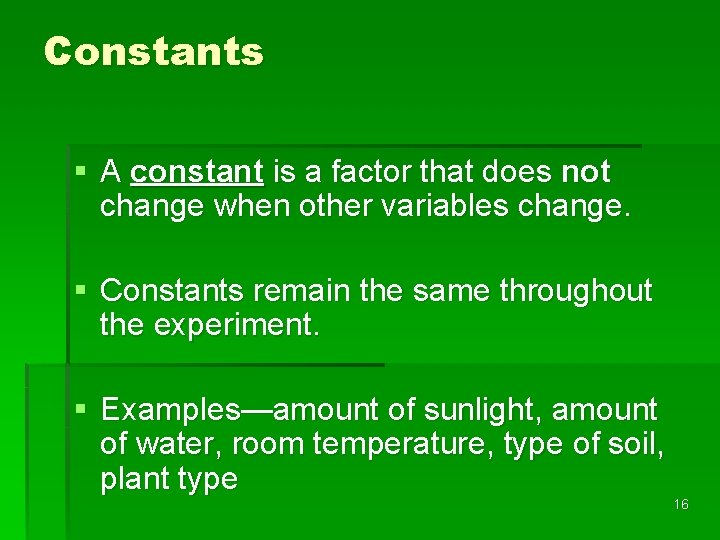
Constants § A constant is a factor that does not change when other variables change. § Constants remain the same throughout the experiment. § Examples—amount of sunlight, amount of water, room temperature, type of soil, plant type 16

Control § A control is the standard by which the test results can be compared. § One plant has no fertilizer. This plant is the control. § Ex. Three fertilized plants grow between 2 -3 cms. VS. The unfertilized plant grows 1. 5 cms. 17

Are science and technology the same? § Science is acquiring knowledge. § Technology is the application of science to help people. Sweet 80’s Picture 18

CHAPTER 1: THE NATURE OF SCIENCE Section 2: Standards of Measurement 19

A standard is an exact quantity that people agree to use for comparison. English Measurement System (U. S. A. ) § Milk gallon § Lumber foot § Potatoes pound Metric (Other Nations) § Based on multiples of 10 and developed in the late 1700’s. § Milk→ Liter § Lumber→Meter § Potatoes→ Kilogram 20

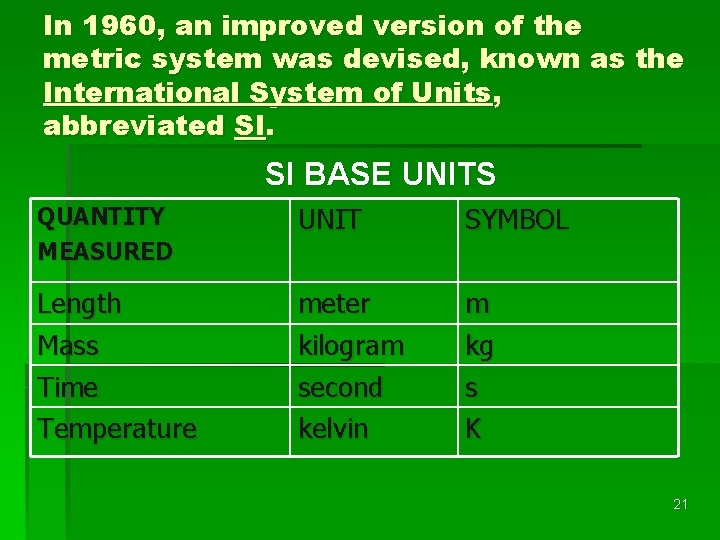
In 1960, an improved version of the metric system was devised, known as the International System of Units, abbreviated SI. SI BASE UNITS QUANTITY MEASURED UNIT SYMBOL Length Mass Time Temperature meter kilogram second kelvin m kg s K 21

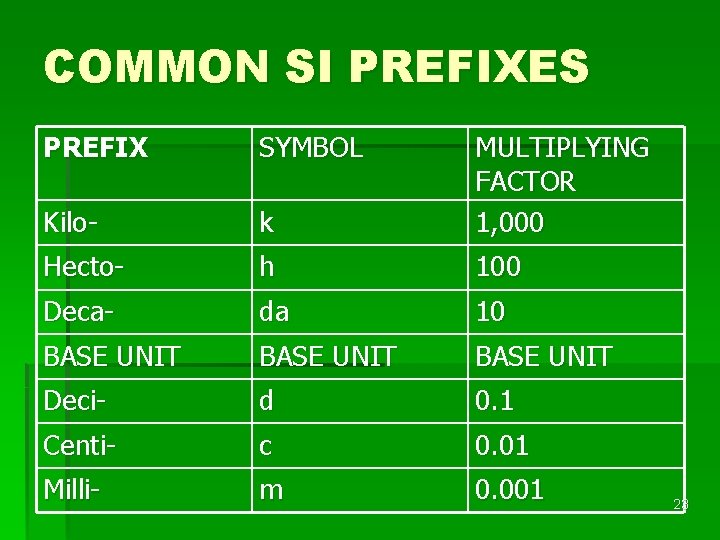
SI PREFIXES are easy to use, because they are based on multiples of 10. § The prefix kilomeans “ 1, 000” § The prefix decimeans “one-tenth” § 1 kilometer = 1, 000 meters § 1 kilogram = 1, 000 grams § 1 decimeter = one-tenth of a meter (0. 1 m) § 1 decigram = one-tenth of a gram (0. 1 g) 22

COMMON SI PREFIXES PREFIX SYMBOL Kilo- k MULTIPLYING FACTOR 1, 000 Hecto- h 100 Deca- da 10 BASE UNIT Deci- d 0. 1 Centi- c 0. 01 Milli- m 0. 001 23

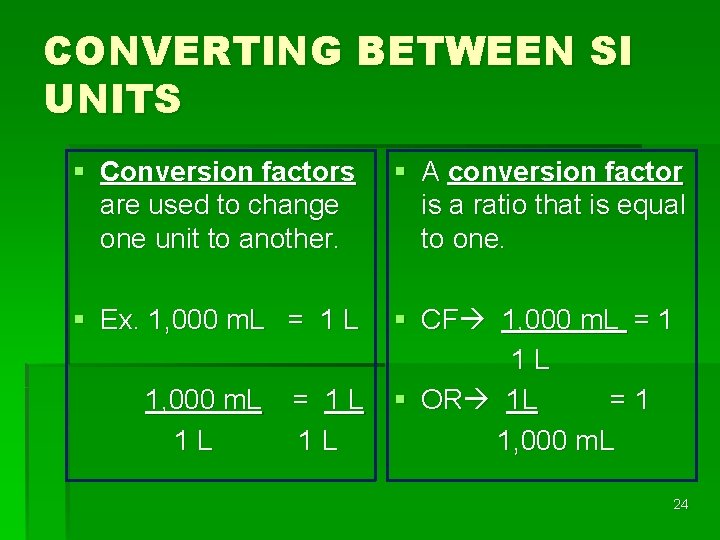
CONVERTING BETWEEN SI UNITS § Conversion factors are used to change one unit to another. § A conversion factor is a ratio that is equal to one. § Ex. 1, 000 m. L = 1 L § CF 1, 000 m. L = 1 1 L § OR 1 L =1 1, 000 m. L 1 L = 1 L 1 L 24

To convert units, you multiply by the appropriate conversion factor. § Ex. 1. 255 L = ? m. L § 1. 255 L x 1, 000 m. L = 1 L 1, 255 m. L 25

MEASURING DISTANCE § The SI unit of length is the meter, m. § Length is measured as the distance between 2 points. 26

The size of the unit you measure with will depend on the size of the object being measured. § Distance from home to school = km § Length of your pencil = cm 27

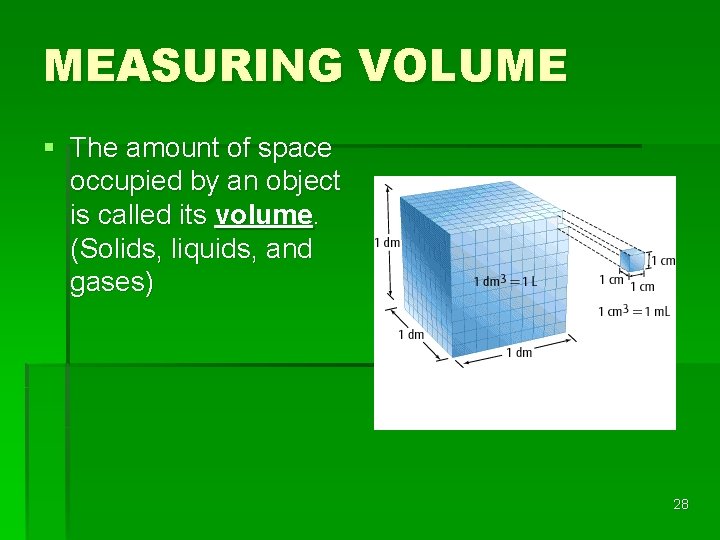
MEASURING VOLUME § The amount of space occupied by an object is called its volume. (Solids, liquids, and gases) 28

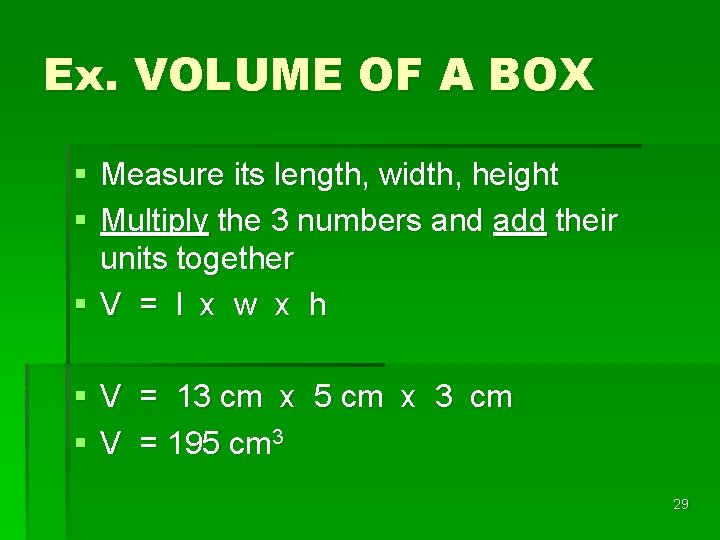
Ex. VOLUME OF A BOX § Measure its length, width, height § Multiply the 3 numbers and add their units together §V = l x w x h § V = 13 cm x 5 cm x 3 cm § V = 195 cm 3 29

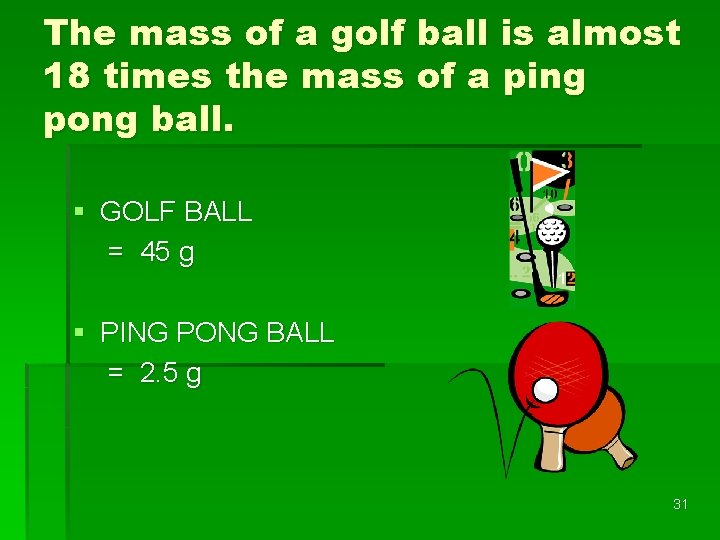
Measuring Matter Mass is a measurement of the quantity of matter in an object. § A table-tennis (ping pong) ball and a golf ball have about the same volume. § The golf ball has more mass. 30

The mass of a golf ball is almost 18 times the mass of a ping pong ball. § GOLF BALL = 45 g § PING PONG BALL = 2. 5 g 31

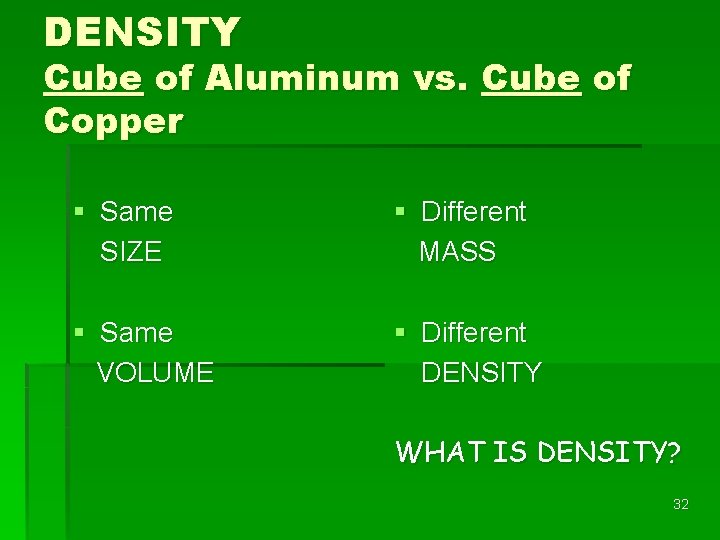
DENSITY Cube of Aluminum vs. Cube of Copper § Same SIZE § Different MASS § Same VOLUME § Different DENSITY WHAT IS DENSITY? 32

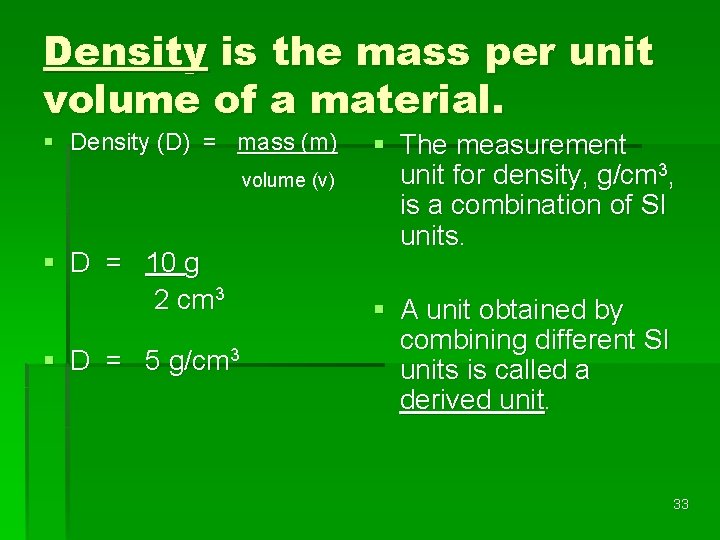
Density is the mass per unit volume of a material. § Density (D) = mass (m) volume (v) § D = 10 g 2 cm 3 § D = 5 g/cm 3 § The measurement unit for density, g/cm 3, is a combination of SI units. § A unit obtained by combining different SI units is called a derived unit. 33

TIME § Time is the interval between 2 events. § The SI unit for time is the second. 34

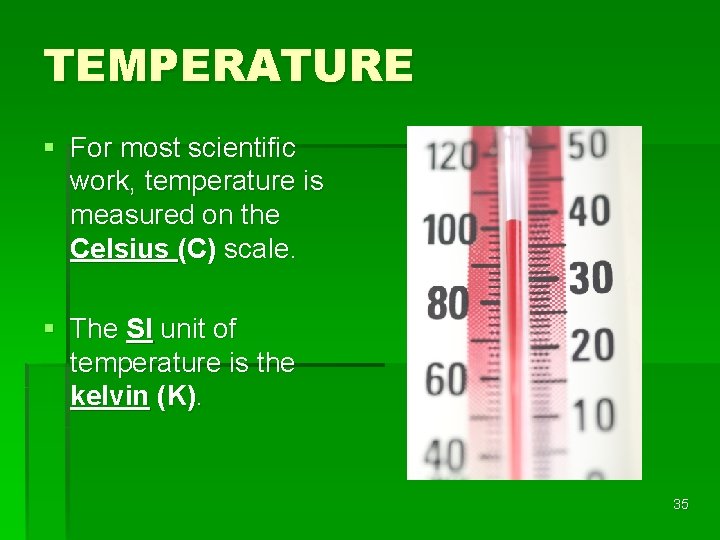
TEMPERATURE § For most scientific work, temperature is measured on the Celsius (C) scale. § The SI unit of temperature is the kelvin (K). 35

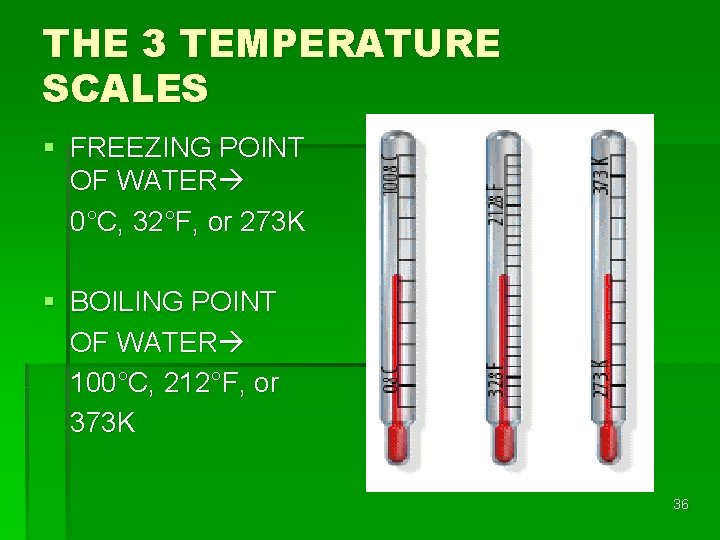
THE 3 TEMPERATURE SCALES § FREEZING POINT OF WATER 0°C, 32°F, or 273 K § BOILING POINT OF WATER 100°C, 212°F, or 373 K 36

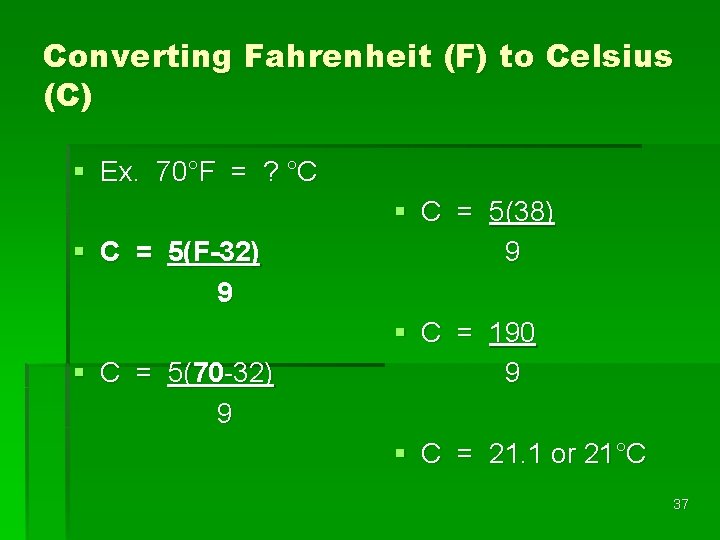
Converting Fahrenheit (F) to Celsius (C) § Ex. 70°F = ? °C § C = 5(F-32) 9 § C = 5(70 -32) 9 § C = 5(38) 9 § C = 190 9 § C = 21. 1 or 21°C 37

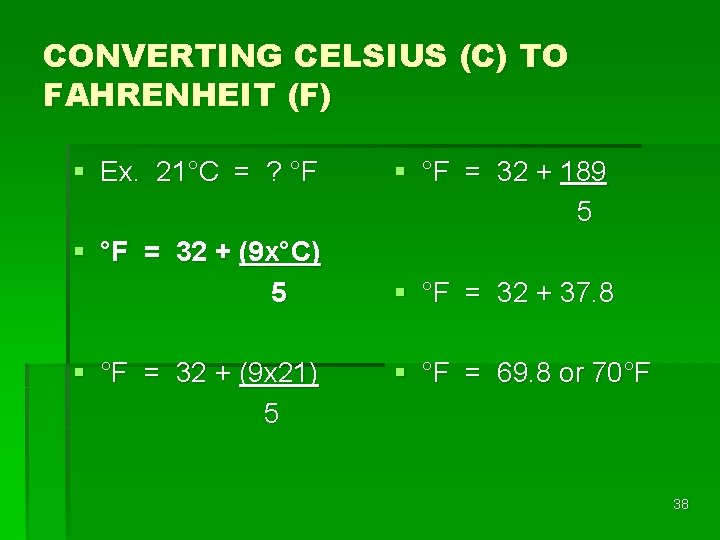
CONVERTING CELSIUS (C) TO FAHRENHEIT (F) § Ex. 21°C = ? °F § °F = 32 + (9 x°C) 5 § °F = 32 + (9 x 21) 5 § °F = 32 + 189 5 § °F = 32 + 37. 8 § °F = 69. 8 or 70°F 38

CHAPTER 1: THE NATURE OF SCIENCE Section 3—Communicating with Graphs 39

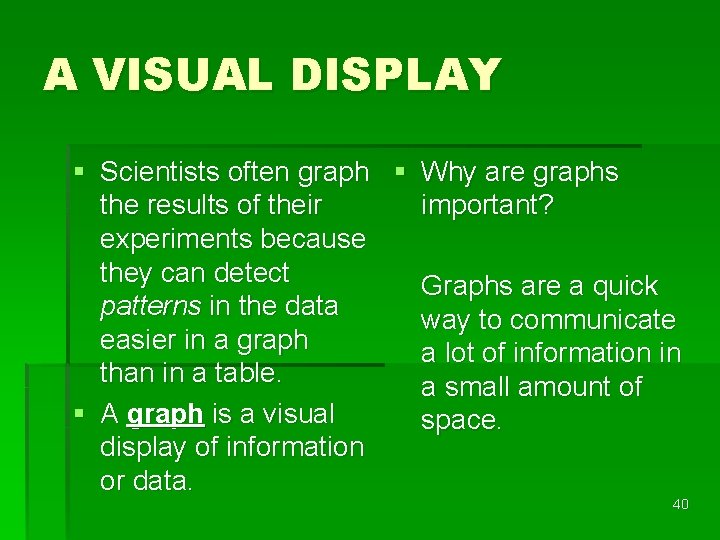
A VISUAL DISPLAY § Scientists often graph § Why are graphs the results of their important? experiments because they can detect Graphs are a quick patterns in the data way to communicate easier in a graph a lot of information in than in a table. a small amount of § A graph is a visual space. display of information or data. 40

There are 3 types of graphs--line, bar, and circle (pie). § Line graphs show a relationship between variables changes over time. 41

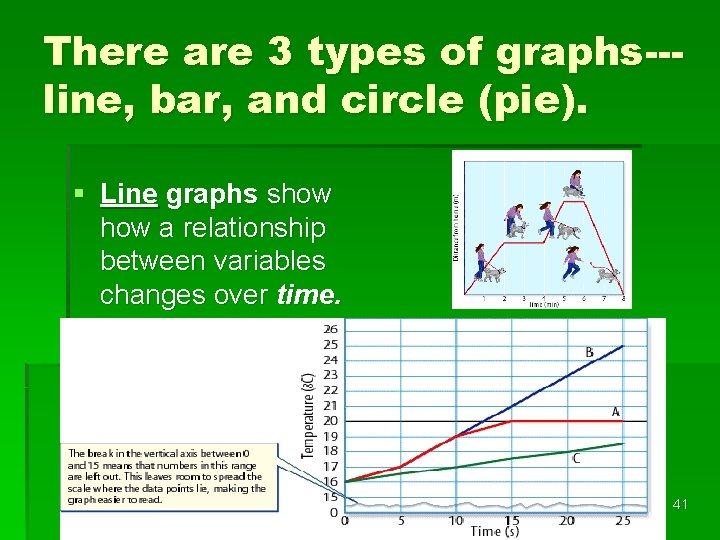
Bar Graphs § A bar graph is useful for comparing information collected by counting. 42

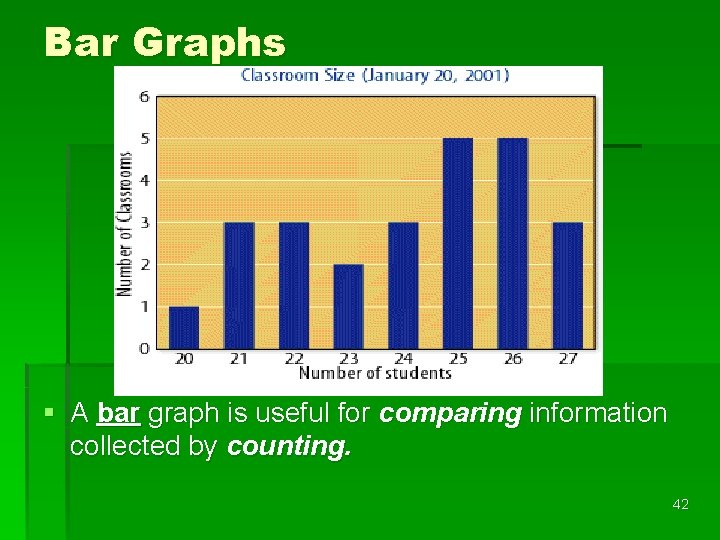
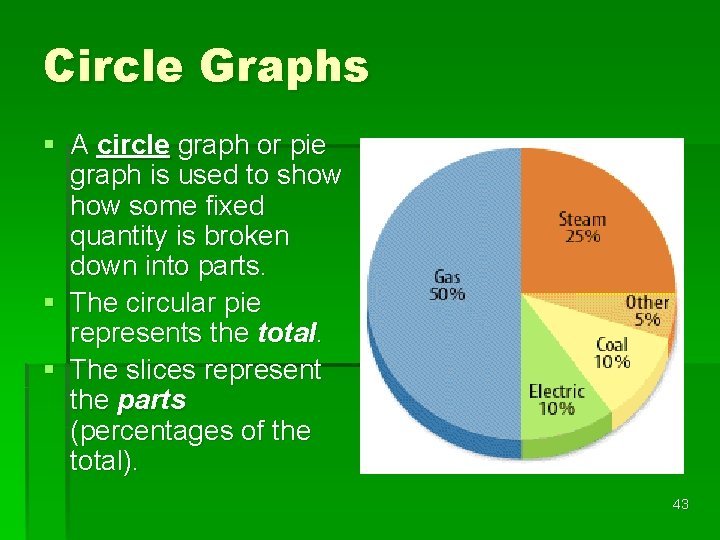
Circle Graphs § A circle graph or pie graph is used to show some fixed quantity is broken down into parts. § The circular pie represents the total. § The slices represent the parts (percentages of the total). 43
 Branch of natural science
Branch of natural science Natural and physical science
Natural and physical science Nature of science chapter 1
Nature of science chapter 1 Section 3 communicating with graphs answer key
Section 3 communicating with graphs answer key Chapter 1 the nature of science study guide answers
Chapter 1 the nature of science study guide answers Nature and nature's laws lay hid in night
Nature and nature's laws lay hid in night Determinace lidské psychiky
Determinace lidské psychiky Physics chapter 2 review
Physics chapter 2 review Chapter 6 physical science
Chapter 6 physical science Chapter 14 physical science test
Chapter 14 physical science test Which travels along a surface separating two media
Which travels along a surface separating two media Physical science jeopardy
Physical science jeopardy Physical science chapter 4 review
Physical science chapter 4 review Chapter 11 physical science
Chapter 11 physical science Chapter 14 review physical science
Chapter 14 review physical science Conceptual physical science practice sheet chapter 2
Conceptual physical science practice sheet chapter 2 Physical science chapter 5 review
Physical science chapter 5 review As your room gets messier day by day, entropy is
As your room gets messier day by day, entropy is Chapter 16 review physical science
Chapter 16 review physical science Math is my favorite subject
Math is my favorite subject Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton
Tư thế worm breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Giọng cùng tên là
Giọng cùng tên là