PHYSICAL SCIENCE CHAPTER 1 METHODS OF SCIENCE CHAPTER




















- Slides: 44

PHYSICAL SCIENCE CHAPTER 1 METHODS OF SCIENCE

CHAPTER GOALS A. B. C. D. E. Learn to define science. Learn the steps to the scientific method. Learn about Experimental Design. Learn and use the SI system units. Density

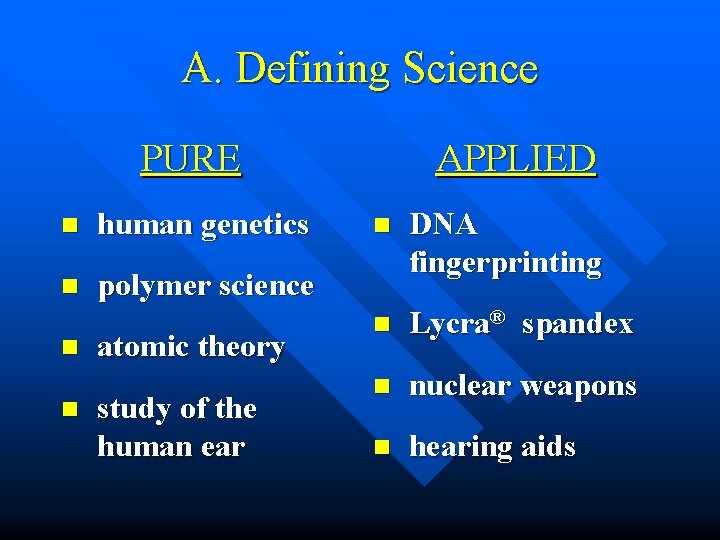
A. Defining Science n Pure Science – research that adds to the body of scientific knowledge – has no practical use n Applied Science (Technology) – the practical application of scientific knowledge

A. Defining Science PURE n human genetics n polymer science n n atomic theory study of the human ear APPLIED n DNA fingerprinting n Lycra® spandex n nuclear weapons n hearing aids

A. Defining Science n Life Science – the study of living organisms n Earth Science – the study of Earth and space n Physical Science – the study of matter and energy – chemistry & physics

B. SCIENTIFIC METHOD What is the scientific method? It is a way to solve a problem!

B. SCIENTIFIC METHOD STEP ONE State the problem. You must identify a problem or question before beginning the scientific method.

B. SCIENTIFIC METHOD STEP TWO Research the problem. Try to find out the details of the problem. Do some investigating.

B. SCIENTIFIC METHOD STEP THREE Form a hypothesis. A hypothesis is an educated guess.

B. SCIENTIFIC METHOD STEP FOUR Test the hypothesis. Scientist test their hypothesis through gathering data and performing experiments.

B. SCIENTIFIC METHOD STEP FIVE Draw conclusions from the data or experiments.

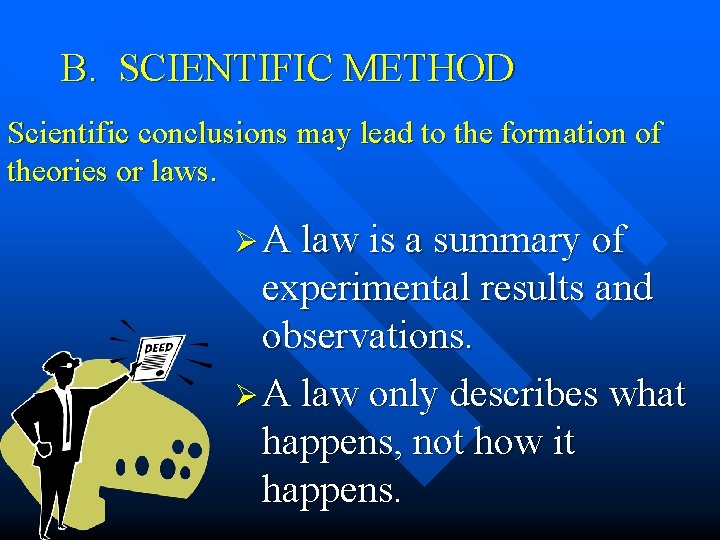
B. SCIENTIFIC METHOD Scientific conclusions may lead to the formation of theories or laws. Ø A law is a summary of experimental results and observations. Ø A law only describes what happens, not how it happens.

B. SCIENTIFIC METHOD Ø An explanation of why things work the way they do is called a theory. A theory can be used to predict the results of future experiments.

B. SCIENTIFIC METHOD Theories and laws are well-accepted by scientists, but. . . THEY ARE NOT SET IN STONE! They are revised when new information is discovered.

REVIEW ØWhy do we use the scientific method? ØWhat is the first step? ØWhat is a hypothesis? ØWhat do you do if your hypothesis is wrong?

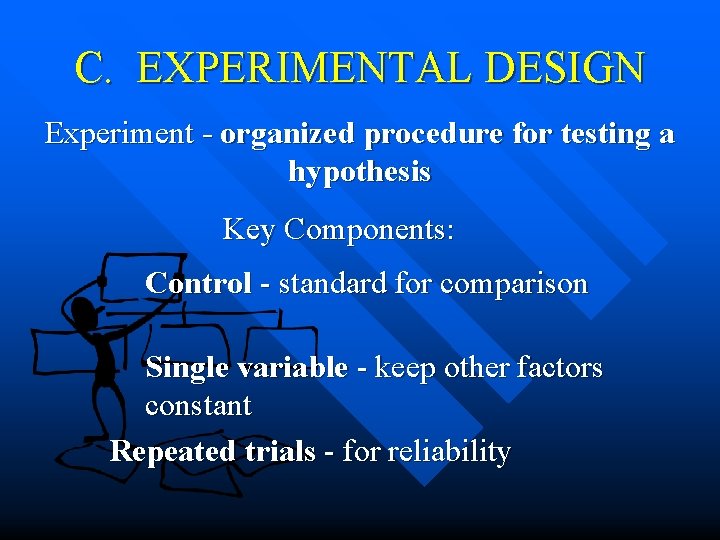
C. EXPERIMENTAL DESIGN Experiment - organized procedure for testing a hypothesis Key Components: Control - standard for comparison Single variable - keep other factors constant Repeated trials - for reliability

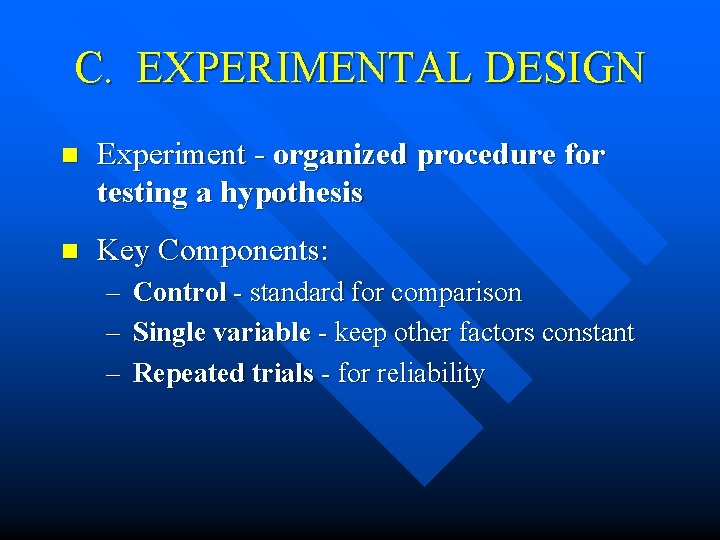
C. EXPERIMENTAL DESIGN n Experiment - organized procedure for testing a hypothesis n Key Components: – Control - standard for comparison – Single variable - keep other factors constant – Repeated trials - for reliability

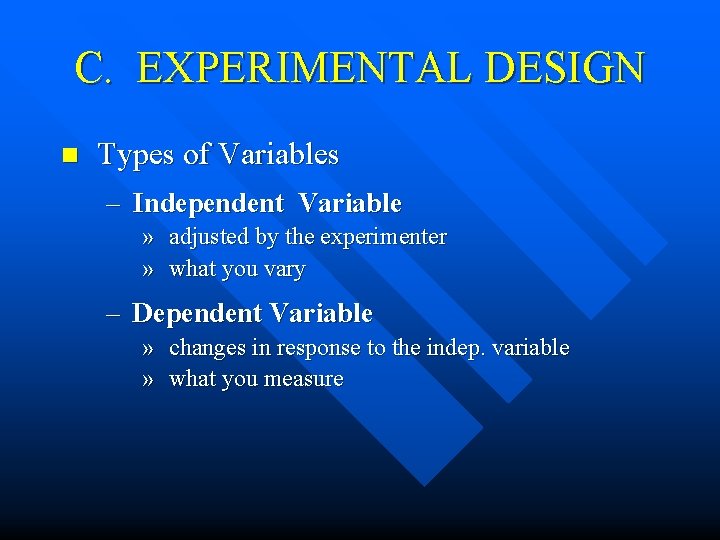
C. EXPERIMENTAL DESIGN n Types of Variables – Independent Variable » adjusted by the experimenter » what you vary – Dependent Variable » changes in response to the indep. variable » what you measure

C. EXPERIMENTAL DESIGN n Hypothesis: Storing popcorn in the freezer makes it pop better. n Control: Popcorn stored at room temp.

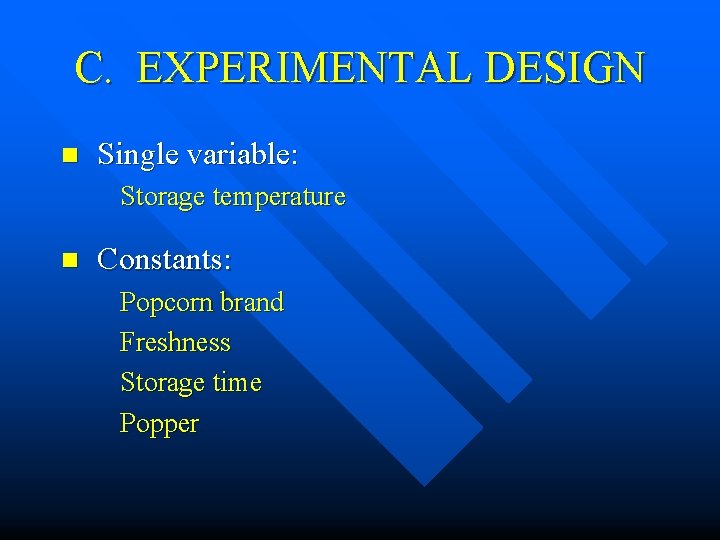
C. EXPERIMENTAL DESIGN n Single variable: Storage temperature n Constants: Popcorn brand Freshness Storage time Popper

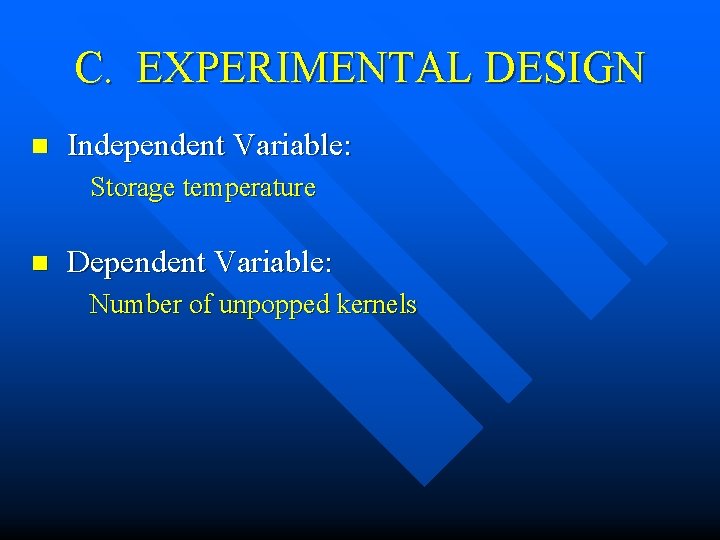
C. EXPERIMENTAL DESIGN n Independent Variable: Storage temperature n Dependent Variable: Number of unpopped kernels

D. SI UNITS AND CONVERSIONS During the 1790’s a group of French scientist came up with the International System of measurement. This system works by combining prefixes and base units.

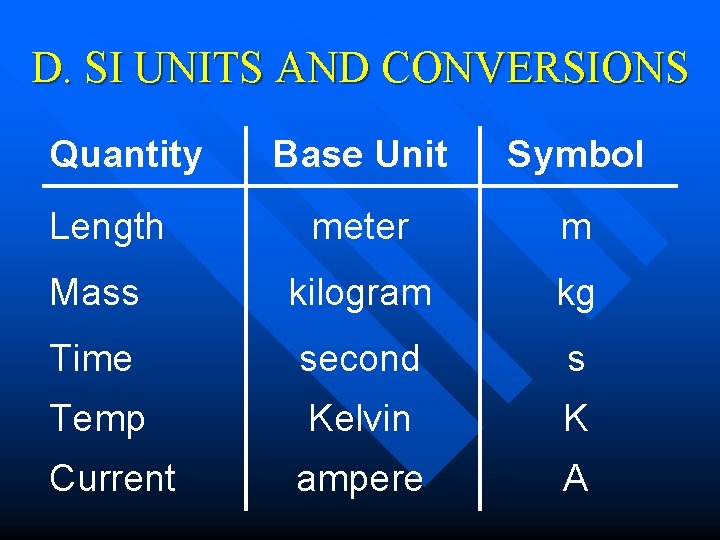
D. SI UNITS AND CONVERSIONS Quantity Base Unit Symbol meter m Mass kilogram kg Time second s Temp Kelvin K ampere A Length Current

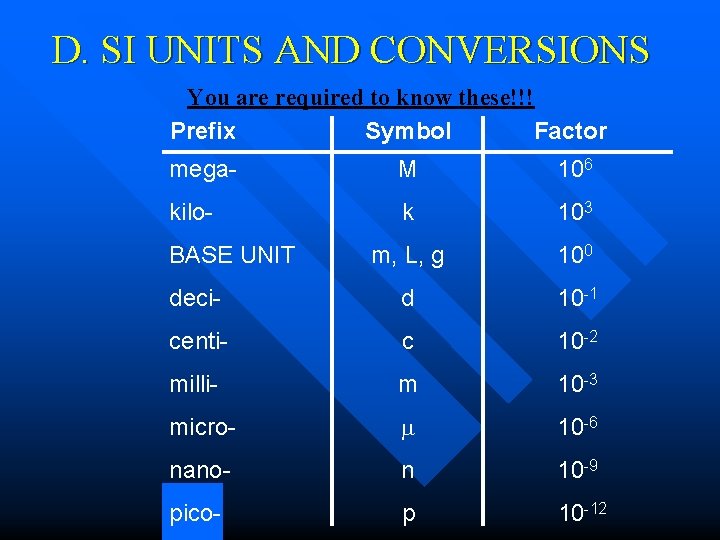
D. SI UNITS AND CONVERSIONS You are required to know these!!! Prefix Symbol Factor mega- M 106 kilo- k 103 m, L, g 100 deci- d 10 -1 centi- c 10 -2 milli- m 10 -3 micro- 10 -6 nano- n 10 -9 pico- p 10 -12 BASE UNIT

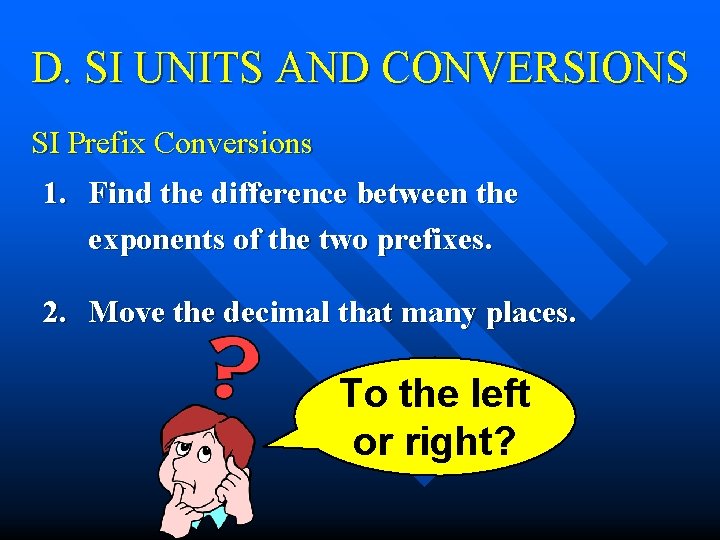
D. SI UNITS AND CONVERSIONS SI Prefix Conversions 1. Find the difference between the exponents of the two prefixes. 2. Move the decimal that many places. To the left or right?

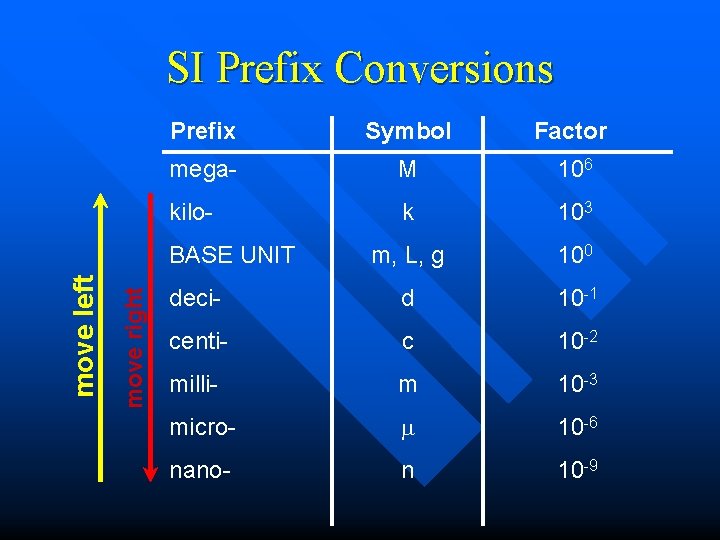
SI Prefix Conversions Prefix Symbol Factor mega- M 106 kilo- k 103 m, L, g 100 deci- d 10 -1 centi- c 10 -2 milli- m 10 -3 micro- 10 -6 nano- n 10 -9 move right move left BASE UNIT

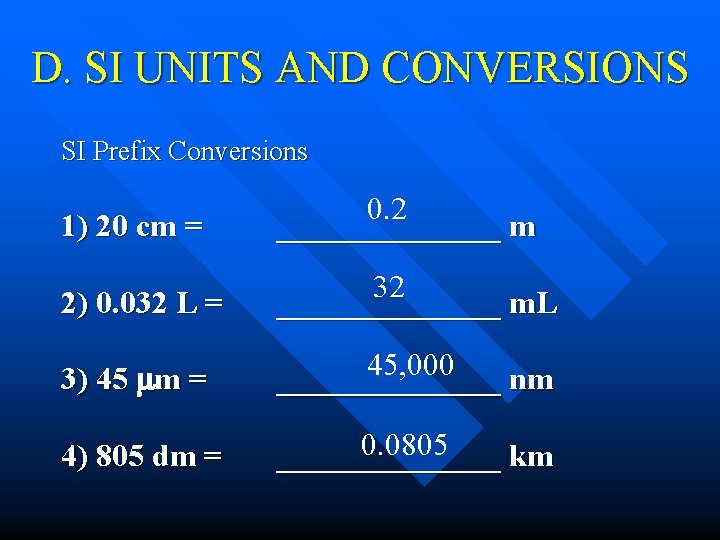
D. SI UNITS AND CONVERSIONS SI Prefix Conversions 1) 20 cm = 0. 2 _______ m 2) 0. 032 L = 32 _______ m. L 3) 45 m = 45, 000 _______ nm 4) 805 dm = 0. 0805 _______ km

D. SI UNITS AND CONVERSIONS Derived Units n n Combination of base units. Volume - length 1 cm 3 = 1 m. L 1 dm 3 = 1 L

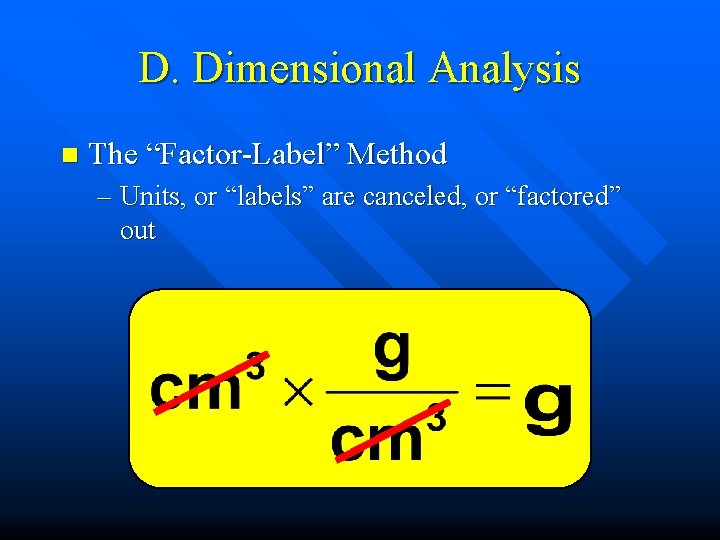
D. Dimensional Analysis n The “Factor-Label” Method – Units, or “labels” are canceled, or “factored” out

D. Dimensional Analysis n Steps: 1. Identify starting & ending units. 2. Line up conversion factors so units cancel. 3. Multiply all top numbers & divide by each bottom number. 4. Check units & answer.

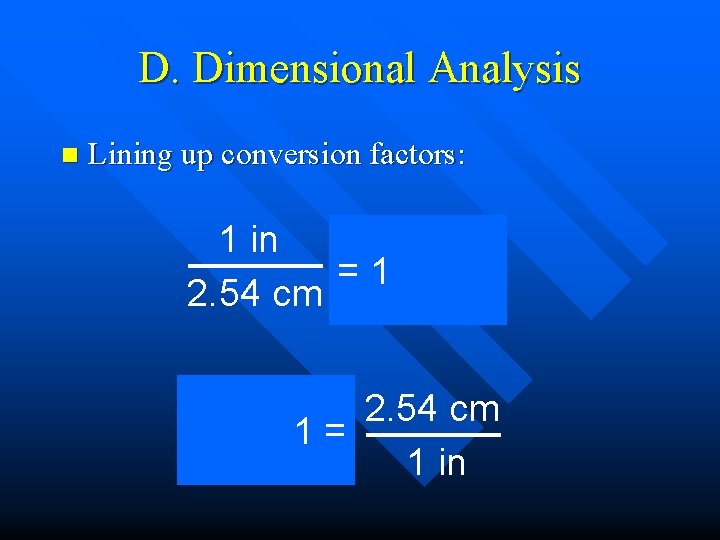
D. Dimensional Analysis n Lining up conversion factors: 1 in = 2. 54 cm =1 2. 54 cm 1 in = 2. 54 cm 1= 1 in

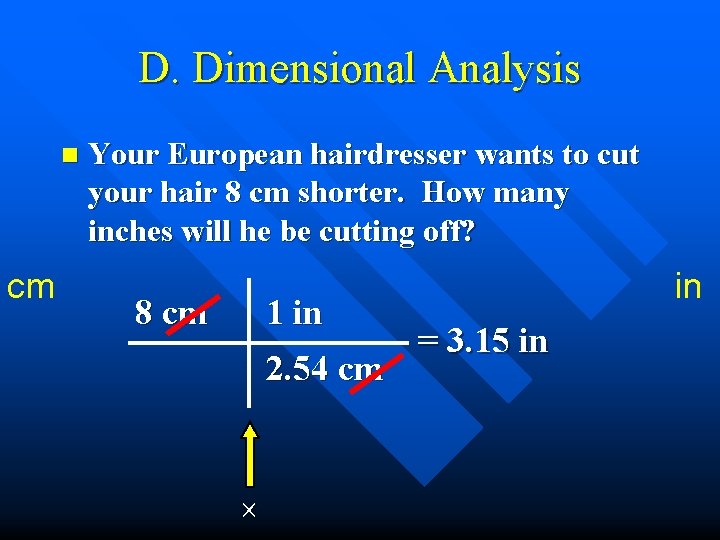
D. Dimensional Analysis n cm Your European hairdresser wants to cut your hair 8 cm shorter. How many inches will he be cutting off? 8 cm 1 in 2. 54 cm in = 3. 15 in

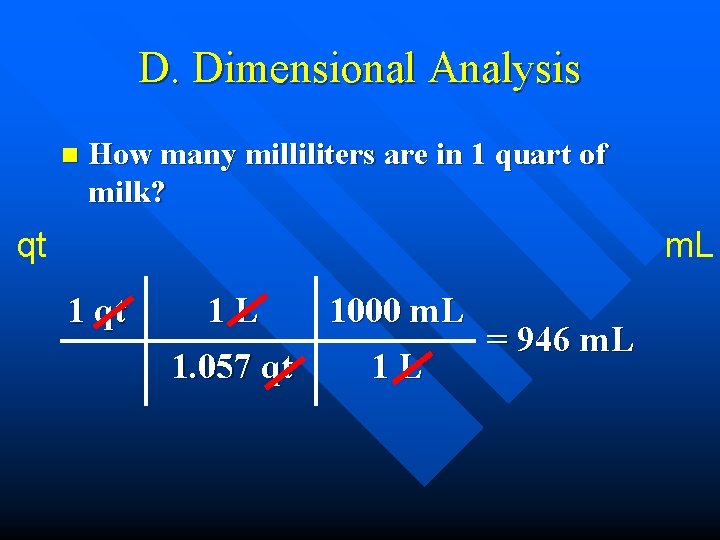
D. Dimensional Analysis n How many milliliters are in 1 quart of milk? qt m. L 1 qt 1 L 1000 m. L 1. 057 qt 1 L = 946 m. L

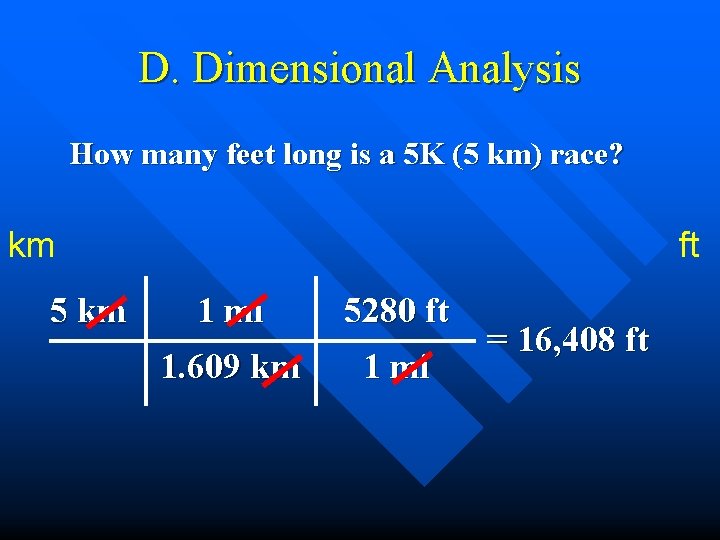
D. Dimensional Analysis How many feet long is a 5 K (5 km) race? km 5 km ft 1 mi 5280 ft 1. 609 km 1 mi = 16, 408 ft

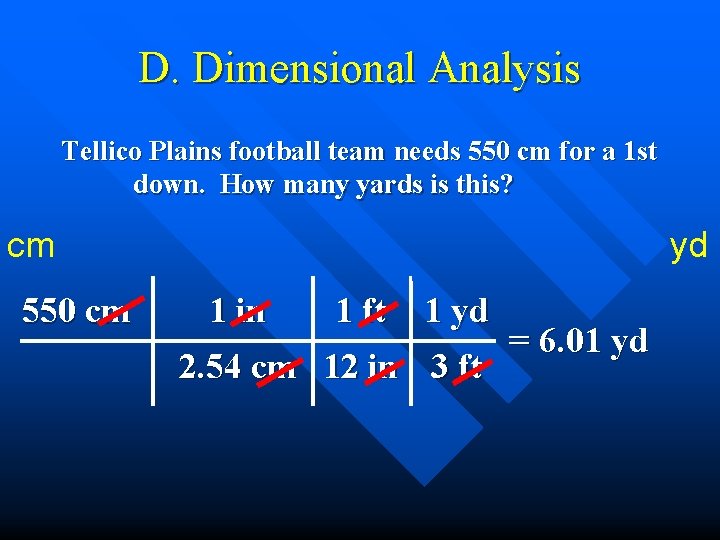
D. Dimensional Analysis Tellico Plains football team needs 550 cm for a 1 st down. How many yards is this? cm 550 cm yd 1 in 1 ft 1 yd 2. 54 cm 12 in 3 ft = 6. 01 yd

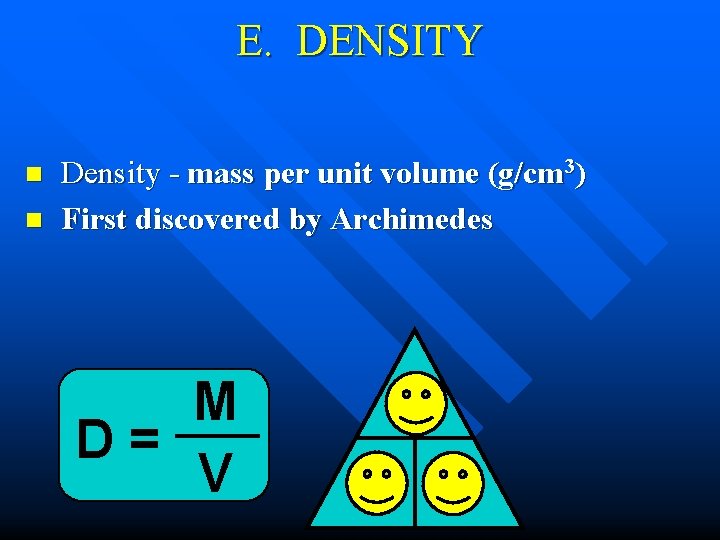
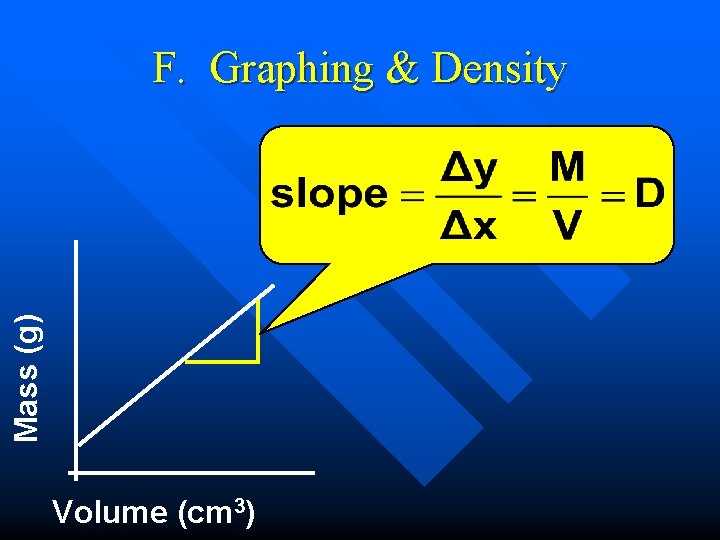
E. DENSITY n n Density - mass per unit volume (g/cm 3) First discovered by Archimedes M D= V M D V

E. DENSITY Ø An object has a volume of 825 cm 3 and a density of 13. 6 g/cm 3. Find its mass. GIVEN: WORK: V = 825 cm 3 D = 13. 6 g/cm 3 M=? M = DV M = (13. 6 g/cm 3)(825 cm 3) M = 11, 200 g

E. DENSITY Ø A liquid has a density of 0. 87 g/m. L. What volume is occupied by 25 g of the liquid? GIVEN: WORK: D = 0. 87 g/m. L V=? M = 25 g V=M V = D 25 g 0. 87 g/m. L V = 29 m. L

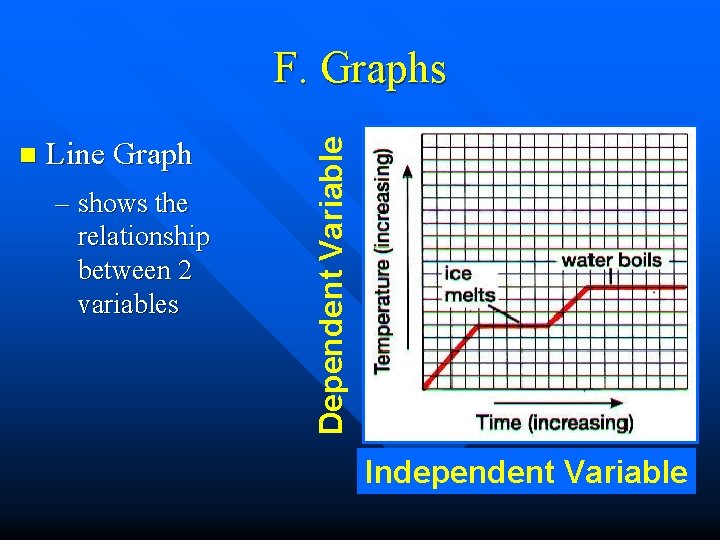
n Line Graph – shows the relationship between 2 variables Dependent Variable F. Graphs Independent Variable

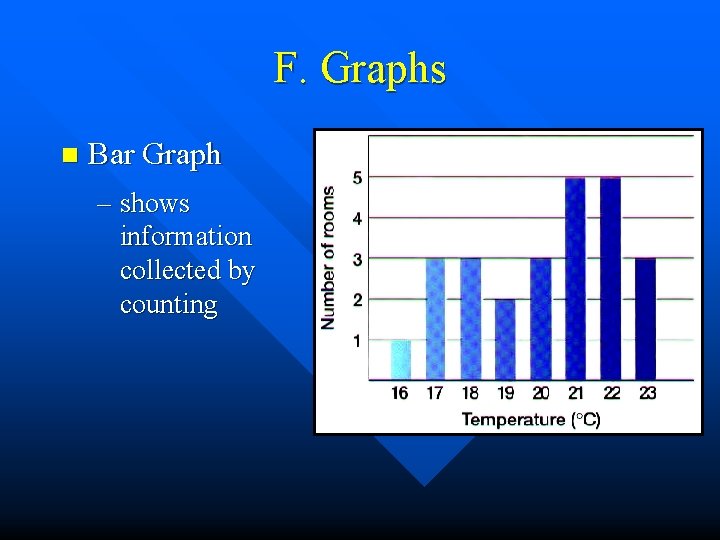
F. Graphs n Bar Graph – shows information collected by counting

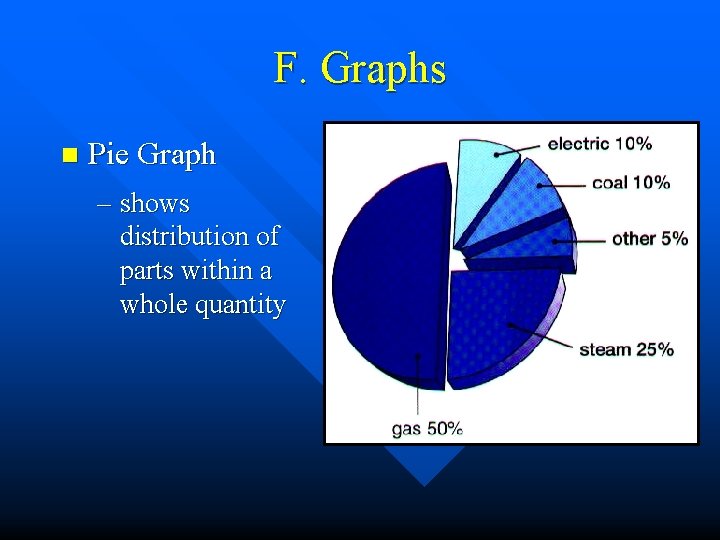
F. Graphs n Pie Graph – shows distribution of parts within a whole quantity

Mass (g) F. Graphing & Density Volume (cm 3)

HERE IS YOUR PROBLEM: Ø How can you drop an egg from the top of the football stadium without breaking the egg? Ø You must follow the rules of the scientific method

HERE IS YOUR PROBLEM: Ø Your design must be easy to open after the egg has been dropped. Ø You may not use food in your design (other than the egg). Ø BYOE (Bring Your Own Egg)