Physical Science ATOMS MOLECULES AND EXTENDED STRUCTURES Review





- Slides: 25

Physical Science ATOMS, MOLECULES AND EXTENDED STRUCTURES

Review: Atomic Structure What is matter? Anything that has mass and takes up space Atom: smallest piece of matter than has the properties of an element Element: substances that are made up entirely of one kind of atom, pure substances with distinct properties that DO NOT vary Scale: size, extent or importance (magnitude) of something relative to something else Just how small is the atom? Lets talk about scale and the atom…. Think about the atoms that make up a grapefruit If each atom were the size of a blueberry, the grapefruit would be the size of the EARTH!!!!!

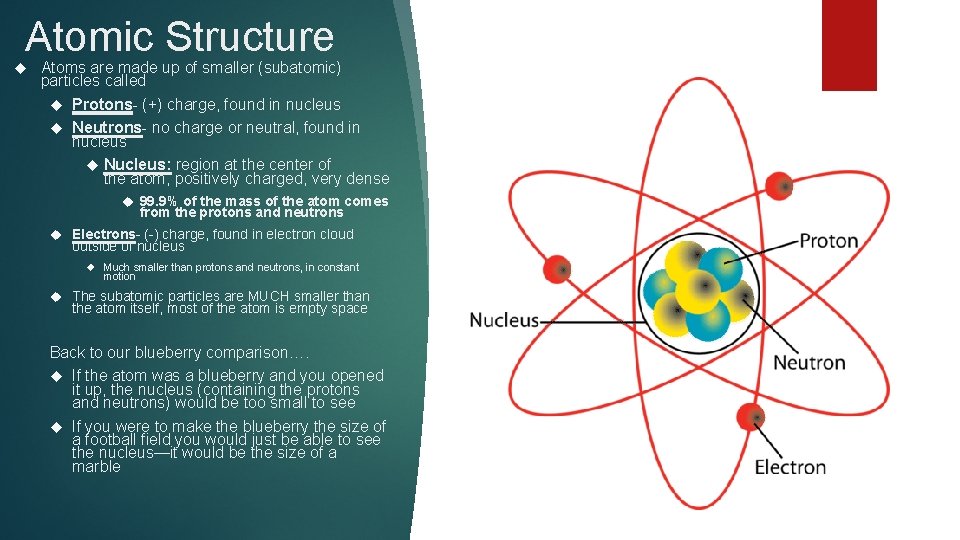
Atomic Structure Atoms are made up of smaller (subatomic) particles called Protons- (+) charge, found in nucleus Neutrons- no charge or neutral, found in nucleus Nucleus: region at the center of the atom, positively charged, very dense 99. 9% of the mass of the atom comes from the protons and neutrons Electrons- (-) charge, found in electron cloud outside of nucleus Much smaller than protons and neutrons, in constant motion The subatomic particles are MUCH smaller than the atom itself, most of the atom is empty space Back to our blueberry comparison…. If the atom was a blueberry and you opened it up, the nucleus (containing the protons and neutrons) would be too small to see If you were to make the blueberry the size of a football field you would just be able to see the nucleus—it would be the size of a marble

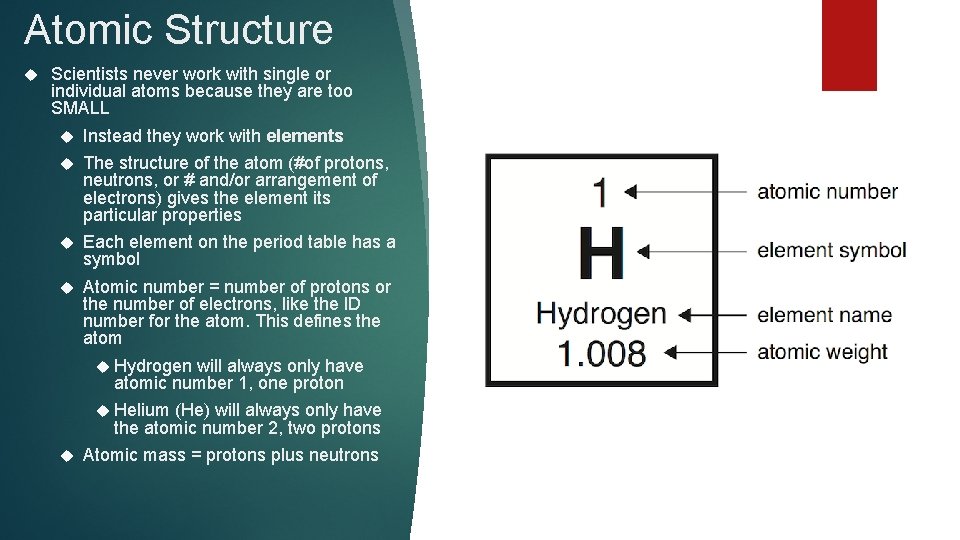
Atomic Structure Scientists never work with single or individual atoms because they are too SMALL Instead they work with elements The structure of the atom (#of protons, neutrons, or # and/or arrangement of electrons) gives the element its particular properties Each element on the period table has a symbol Atomic number = number of protons or the number of electrons, like the ID number for the atom. This defines the atom Hydrogen will always only have atomic number 1, one proton Helium (He) will always only have the atomic number 2, two protons Atomic mass = protons plus neutrons

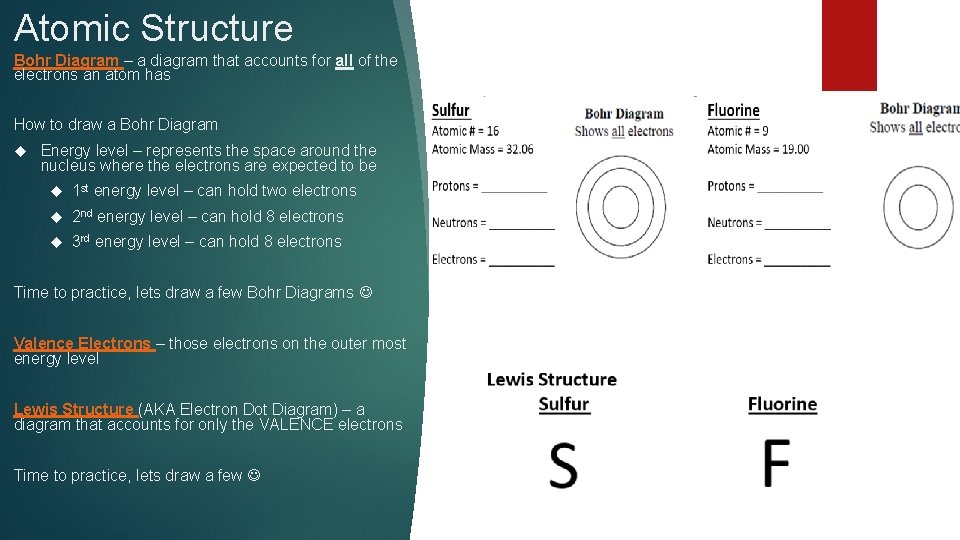
Atomic Structure Bohr Diagram – a diagram that accounts for all of the electrons an atom has How to draw a Bohr Diagram Energy level – represents the space around the nucleus where the electrons are expected to be 1 st energy level – can hold two electrons 2 nd energy level – can hold 8 electrons 3 rd energy level – can hold 8 electrons Time to practice, lets draw a few Bohr Diagrams Valence Electrons – those electrons on the outer most energy level Lewis Structure (AKA Electron Dot Diagram) – a diagram that accounts for only the VALENCE electrons Time to practice, lets draw a few

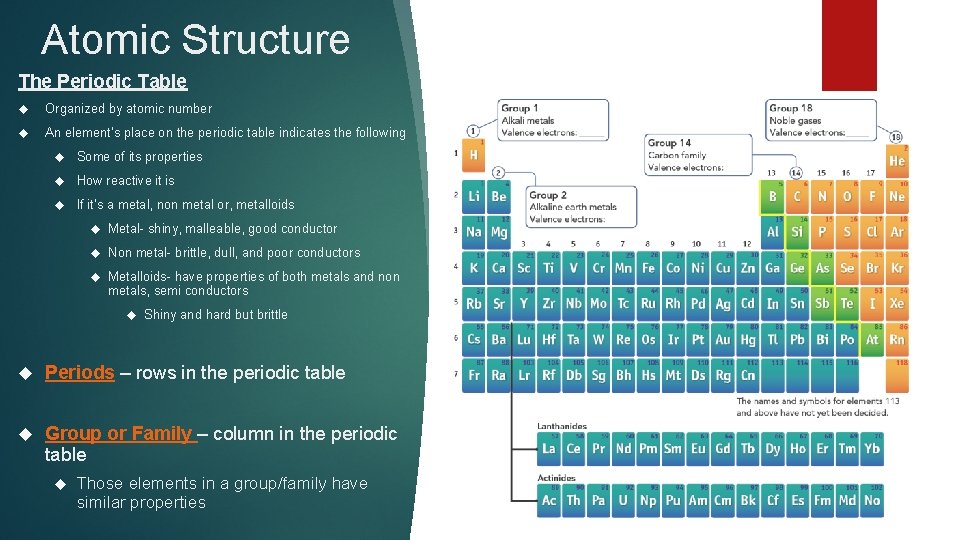
Atomic Structure The Periodic Table Organized by atomic number An element’s place on the periodic table indicates the following Some of its properties How reactive it is If it’s a metal, non metal or, metalloids Metal- shiny, malleable, good conductor Non metal- brittle, dull, and poor conductors Metalloids- have properties of both metals and non metals, semi conductors Shiny and hard but brittle Periods – rows in the periodic table Group or Family – column in the periodic table Those elements in a group/family have similar properties

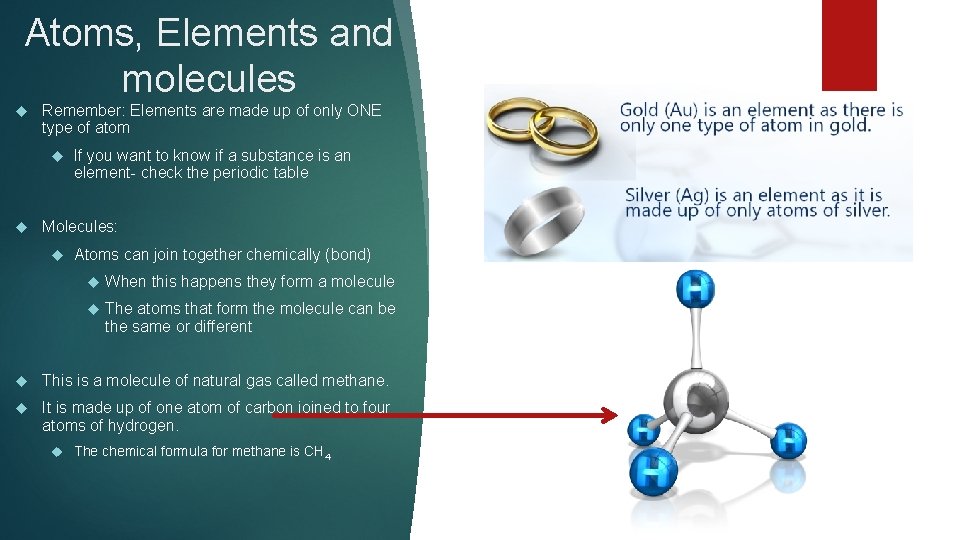
Atoms, Elements and molecules Remember: Elements are made up of only ONE type of atom If you want to know if a substance is an element- check the periodic table Molecules: Atoms can join together chemically (bond) When this happens they form a molecule The atoms that form the molecule can be the same or different This is a molecule of natural gas called methane. It is made up of one atom of carbon joined to four atoms of hydrogen. The chemical formula for methane is CH 4

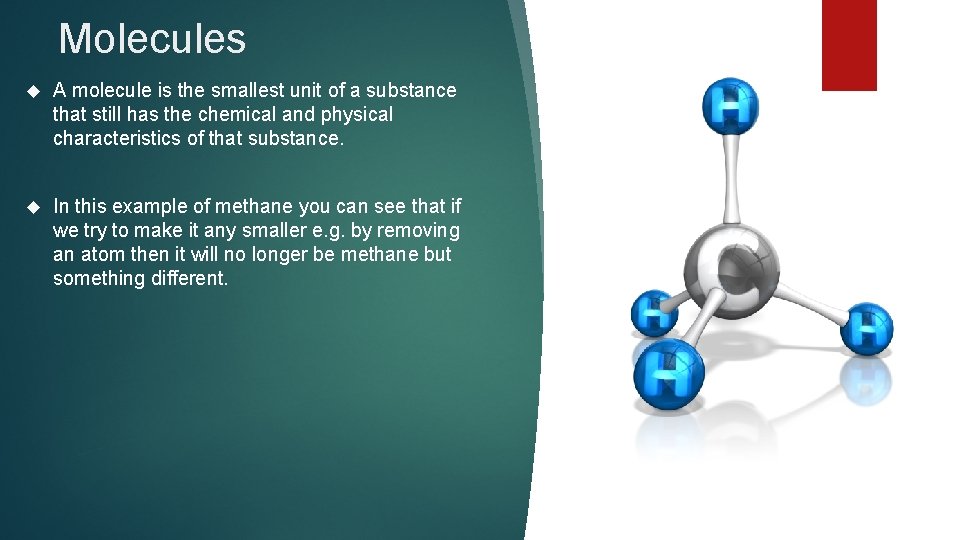
Molecules A molecule is the smallest unit of a substance that still has the chemical and physical characteristics of that substance. In this example of methane you can see that if we try to make it any smaller e. g. by removing an atom then it will no longer be methane but something different.

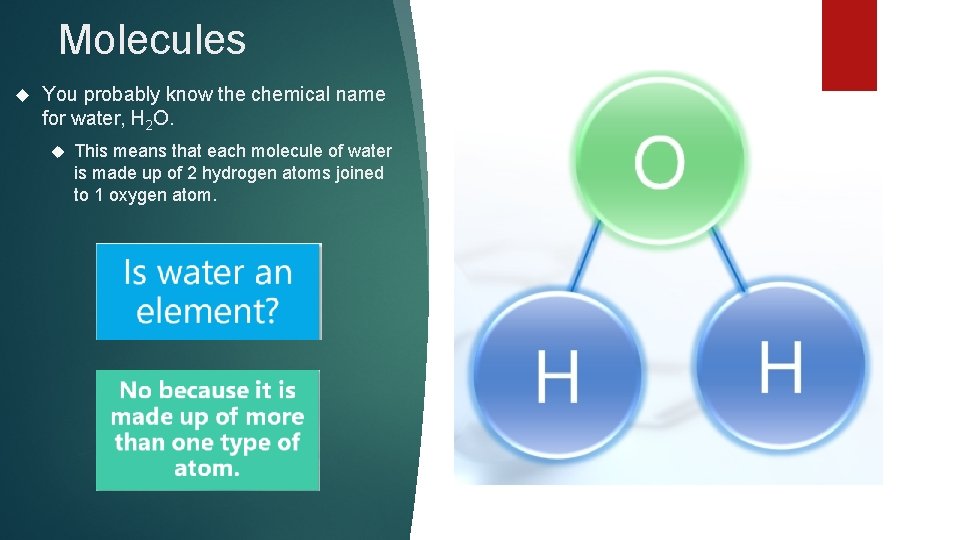
Molecules You probably know the chemical name for water, H 2 O. This means that each molecule of water is made up of 2 hydrogen atoms joined to 1 oxygen atom.

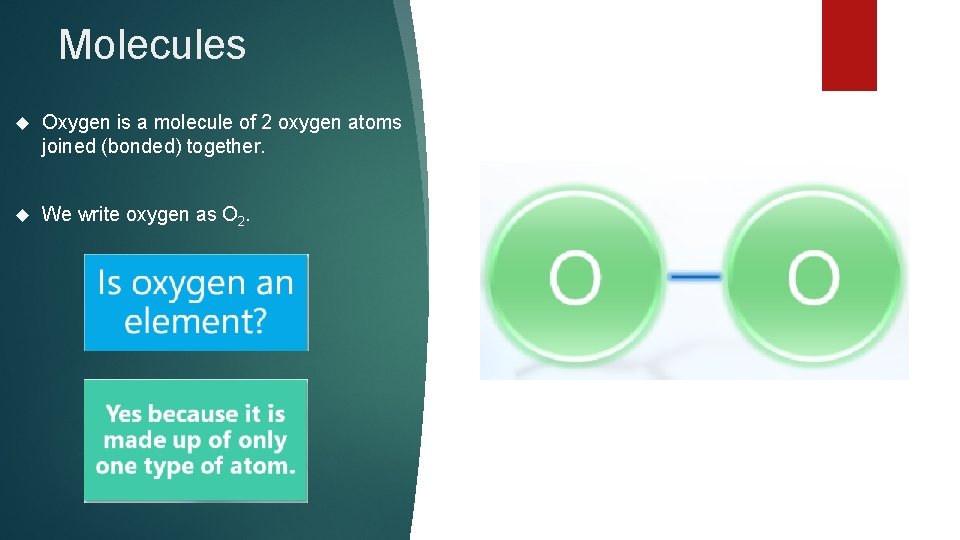
Molecules Oxygen is a molecule of 2 oxygen atoms joined (bonded) together. We write oxygen as O 2.

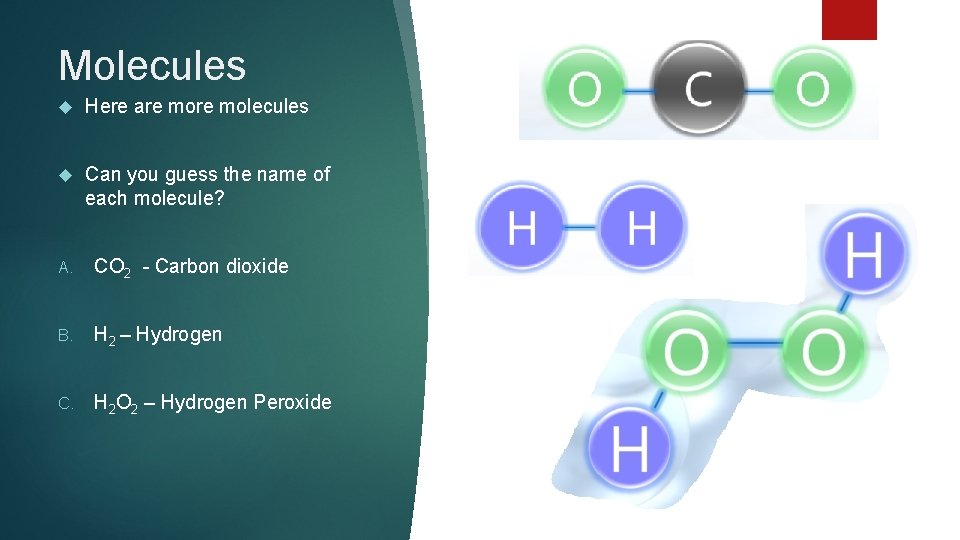
Molecules Here are molecules Can you guess the name of each molecule? A. CO 2 - Carbon dioxide B. H 2 – Hydrogen C. H 2 O 2 – Hydrogen Peroxide

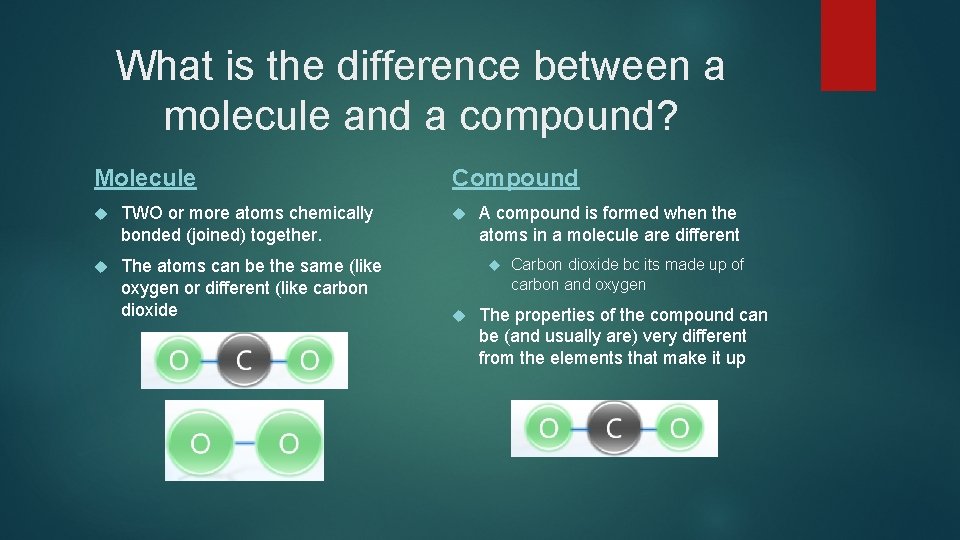
What is the difference between a molecule and a compound? Molecule TWO or more atoms chemically bonded (joined) together. The atoms can be the same (like oxygen or different (like carbon dioxide Compound A compound is formed when the atoms in a molecule are different Carbon dioxide bc its made up of carbon and oxygen The properties of the compound can be (and usually are) very different from the elements that make it up

Compounds Sodium chloride (Na. Cl) is more commonly called salt. It is a compound made from sodium and chlorine. Properties of Salt: Solid White Dissolves in water Has crystal structure Properties of Sodium Very reactive metal Reacts with water to produce hydrogen gas and sodium hydroxide (drain cleaner) Used in street lights Properties of Chlorine (Cl) Green poisonous gas Used in WW 1 in gas attacks Used to kill germs in swimming pools

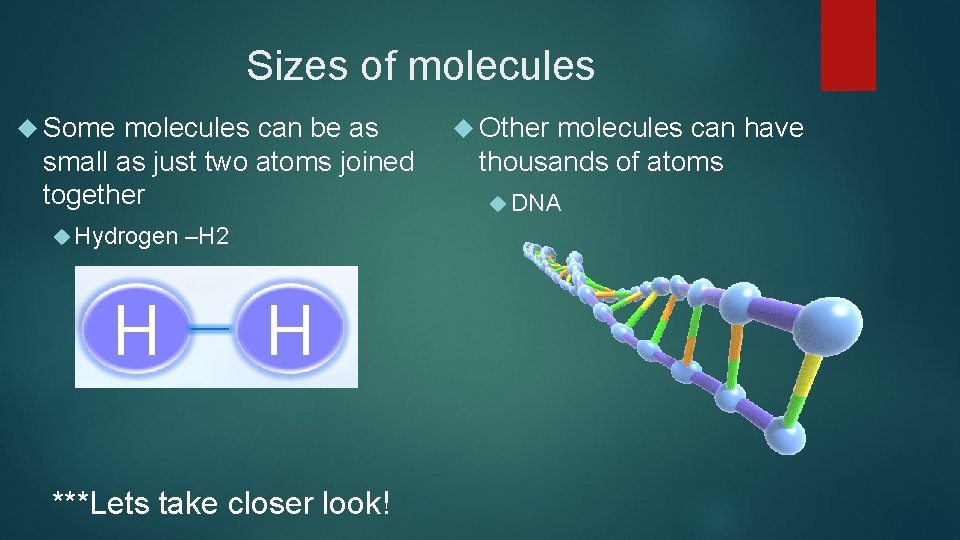
Sizes of molecules Some molecules can be as small as just two atoms joined together Hydrogen –H 2 ***Lets take closer look! Other molecules can have thousands of atoms DNA

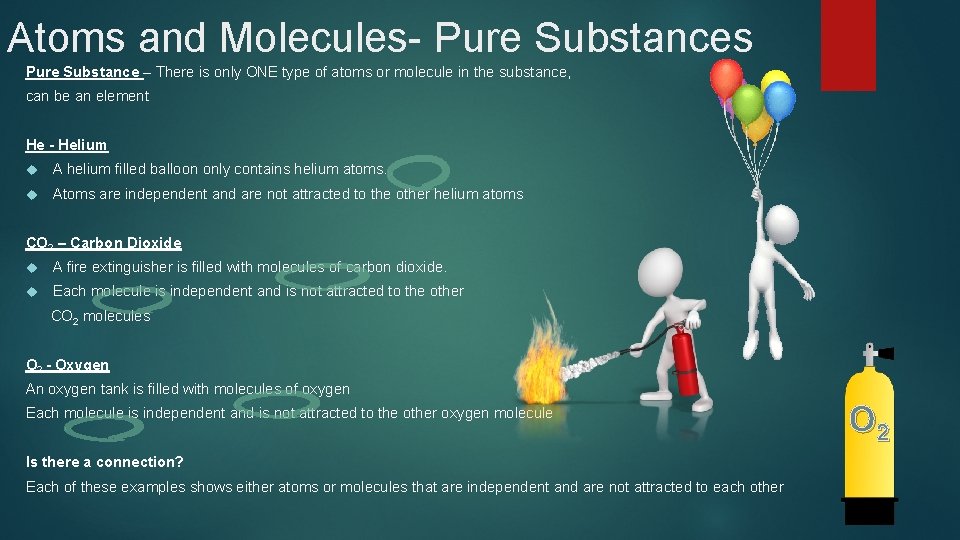
Atoms and Molecules- Pure Substances Pure Substance – There is only ONE type of atoms or molecule in the substance, can be an element He - Helium A helium filled balloon only contains helium atoms. Atoms are independent and are not attracted to the other helium atoms CO 2 – Carbon Dioxide A fire extinguisher is filled with molecules of carbon dioxide. Each molecule is independent and is not attracted to the other CO 2 molecules O 2 - Oxygen An oxygen tank is filled with molecules of oxygen Each molecule is independent and is not attracted to the other oxygen molecule Is there a connection? Each of these examples shows either atoms or molecules that are independent and are not attracted to each other O 2

Molecules – made up of repeating subunits Carbon Basis of all life on earth-EVERY living thing is composed of carbon Can take many different forms depending on how the atoms are arranged

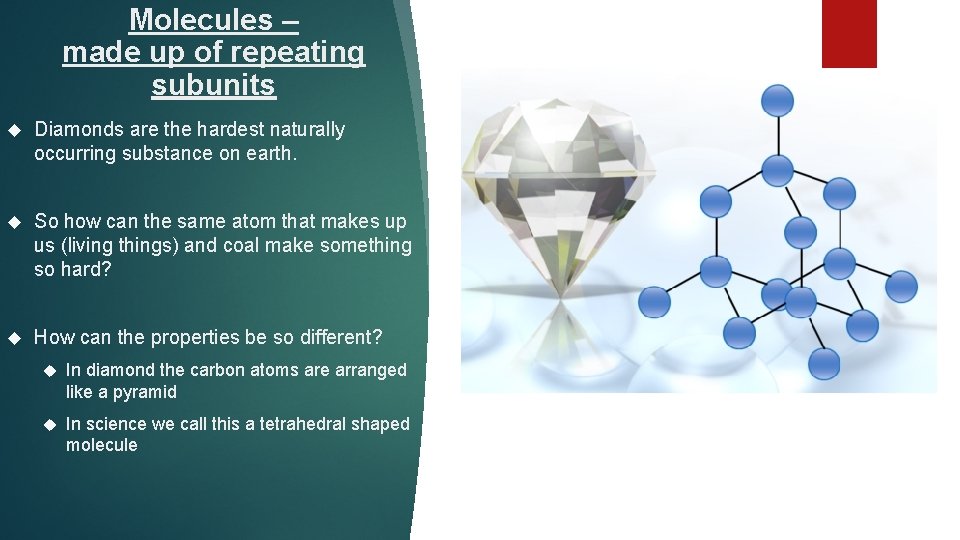
Molecules – made up of repeating subunits Diamonds are the hardest naturally occurring substance on earth. So how can the same atom that makes up us (living things) and coal make something so hard? How can the properties be so different? In diamond the carbon atoms are arranged like a pyramid In science we call this a tetrahedral shaped molecule

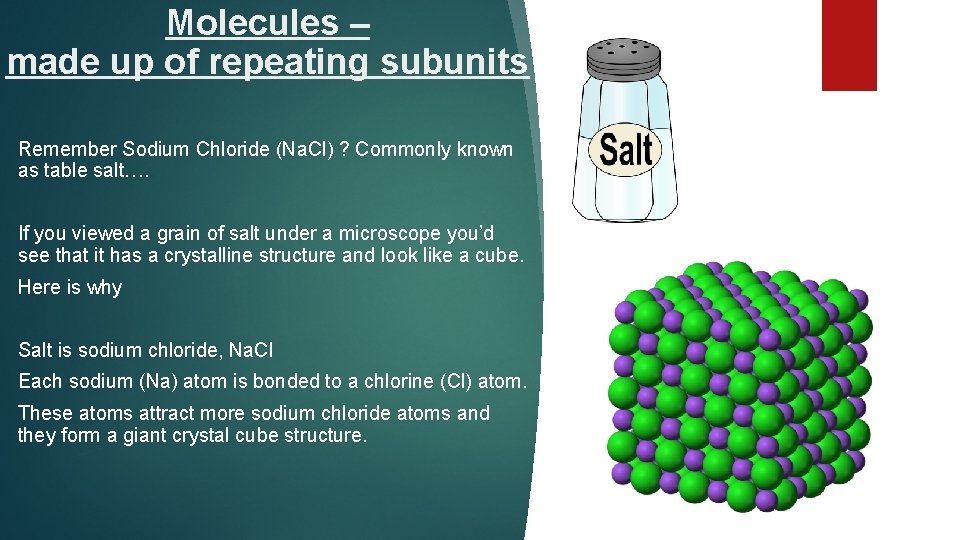
Molecules – made up of repeating subunits Remember Sodium Chloride (Na. Cl) ? Commonly known as table salt…. If you viewed a grain of salt under a microscope you’d see that it has a crystalline structure and look like a cube. Here is why Salt is sodium chloride, Na. Cl Each sodium (Na) atom is bonded to a chlorine (Cl) atom. These atoms attract more sodium chloride atoms and they form a giant crystal cube structure.

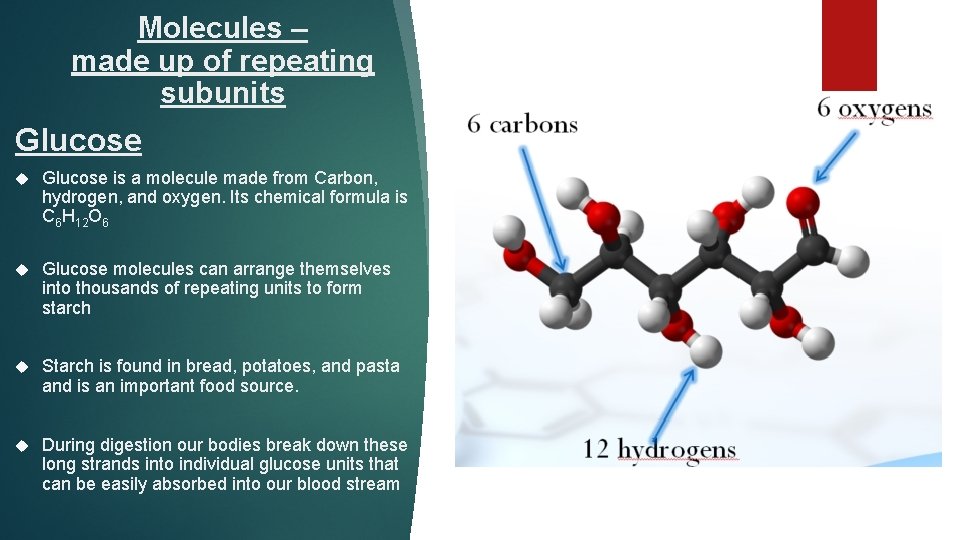
Molecules – made up of repeating subunits Glucose is a molecule made from Carbon, hydrogen, and oxygen. Its chemical formula is C 6 H 12 O 6 Glucose molecules can arrange themselves into thousands of repeating units to form starch Starch is found in bread, potatoes, and pasta and is an important food source. During digestion our bodies break down these long strands into individual glucose units that can be easily absorbed into our blood stream

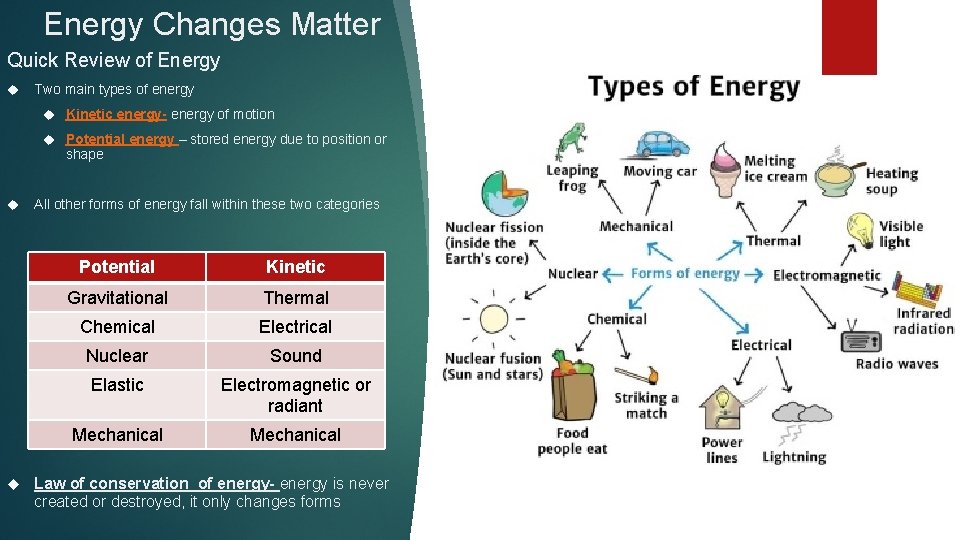
Energy Changes Matter Quick Review of Energy Two main types of energy Kinetic energy- energy of motion Potential energy – stored energy due to position or shape All other forms of energy fall within these two categories Potential Kinetic Gravitational Thermal Chemical Electrical Nuclear Sound Elastic Electromagnetic or radiant Mechanical Law of conservation of energy- energy is never created or destroyed, it only changes forms

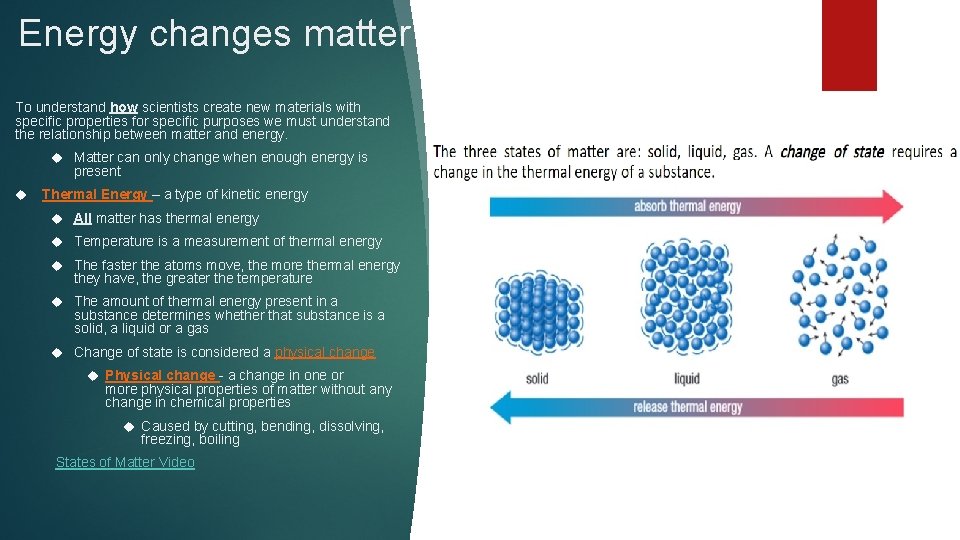
Energy changes matter To understand how scientists create new materials with specific properties for specific purposes we must understand the relationship between matter and energy. Matter can only change when enough energy is present Thermal Energy – a type of kinetic energy All matter has thermal energy Temperature is a measurement of thermal energy The faster the atoms move, the more thermal energy they have, the greater the temperature The amount of thermal energy present in a substance determines whether that substance is a solid, a liquid or a gas Change of state is considered a physical change Physical change - a change in one or more physical properties of matter without any change in chemical properties Caused by cutting, bending, dissolving, freezing, boiling States of Matter Video

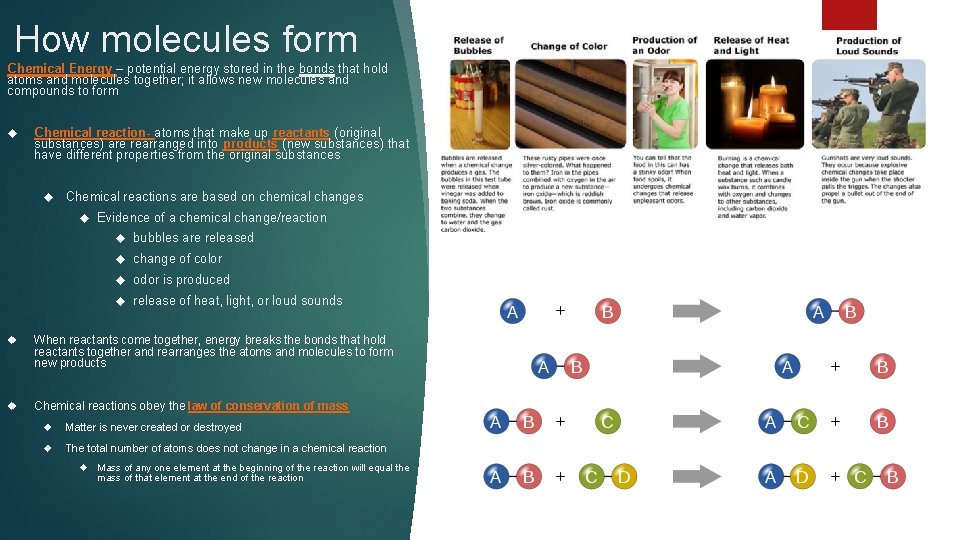
How molecules form Chemical Energy – potential energy stored in the bonds that hold atoms and molecules together; it allows new molecules and compounds to form Chemical reaction- atoms that make up reactants (original substances) are rearranged into products (new substances) that have different properties from the original substances Chemical reactions are based on chemical changes Evidence of a chemical change/reaction bubbles are released change of color odor is produced release of heat, light, or loud sounds When reactants come together, energy breaks the bonds that hold reactants together and rearranges the atoms and molecules to form new products Chemical reactions obey the law of conservation of mass Matter is never created or destroyed The total number of atoms does not change in a chemical reaction Mass of any one element at the beginning of the reaction will equal the mass of that element at the end of the reaction

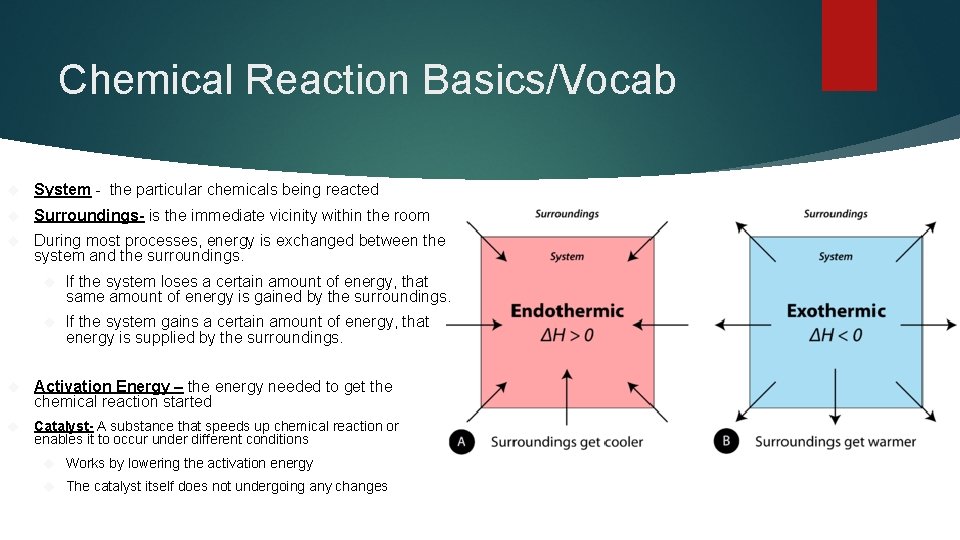
Chemical Reaction Basics/Vocab System - the particular chemicals being reacted Surroundings- is the immediate vicinity within the room During most processes, energy is exchanged between the system and the surroundings. If the system loses a certain amount of energy, that same amount of energy is gained by the surroundings. If the system gains a certain amount of energy, that energy is supplied by the surroundings. Activation Energy – the energy needed to get the chemical reaction started Catalyst- A substance that speeds up chemical reaction or enables it to occur under different conditions Works by lowering the activation energy The catalyst itself does not undergoing any changes

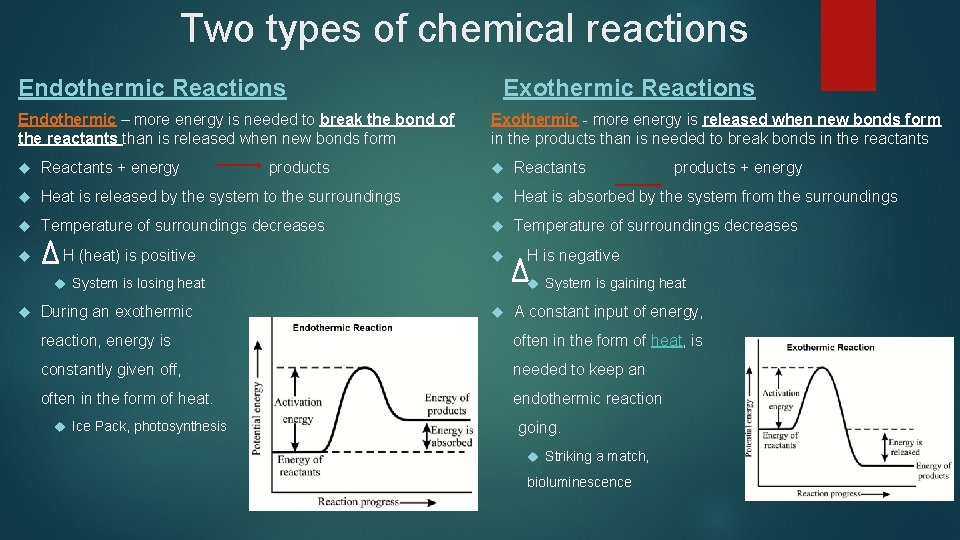
Two types of chemical reactions Endothermic Reactions Endothermic – more energy is needed to break the bond of the reactants than is released when new bonds form Exothermic Reactions Exothermic - more energy is released when new bonds form in the products than is needed to break bonds in the reactants Reactants + energy products Reactants products + energy Heat is released by the system to the surroundings Heat is absorbed by the system from the surroundings Temperature of surroundings decreases H (heat) is positive H is negative System is gaining heat System is losing heat During an exothermic A constant input of energy, reaction, energy is often in the form of heat, is constantly given off, needed to keep an often in the form of heat. endothermic reaction Ice Pack, photosynthesis going. Striking a match, bioluminescence

Endothermic/Exothermic video Exothermic Experiment video