Physical Science Acids Bases p H Memorize quiz

Physical Science Acids, Bases, & p. H

Memorize: quiz Friday!! Common Acids n Hydrochloric acid (HCl) n Sulfuric acid (H 2 SO 4) n Acetic acid (HC 2 H 3 O 2) n Nitric acid (HNO 3) n Citric acid (C 6 H 8 O 7) n n n n Common Bases Sodium bicarbonate (Na. HCO 3) Sodium hydroxide (Na. OH) Potassium hydroxide (KOH) Calcium hydroxide (Ca(OH)2) Magnesium hydroxide (Mg(OH)2) Barium hydroxide (Ba(OH)2) Ammonium hydroxide (NH 4 OH)

Acids n n Substances that dissociate in water to form ions – Hydrogen ions (H+) attach to water to form H 3 O+ (hydronium ion) – process is ionization Examples: n n Battery acid Vinegar Citric acid- lemons, limes Carbonic acid – soda; acid rain

Properties of acids Taste Sour n Conduct electricity n Some are strong, some are weak electrolytes n Indicators (blue litmus turns red) n React with hydroxides (OH-) to form water and salt n Usually starts with an ‘H’ n
n Electrolyte – ions that conduct electricity – When in a solution, the solution is called an electrolytic solution n Nonelectrolyte – molecules that don’t conduct electricity – When in a solution, the solution is called a nonelectrolyte solution

Acids The ones in food are dilute n Concentrated acids are dangerous n They can burn your skin and eyes n Strong acids ionize completely – All the H’s make hydronium n HCl + H 2 O Cl- + H 3 O+ n Makes lots of ions n Can be dangerous n

Bases Ionizes in water to yield hydroxide ions (OH- ) n Has OH- in it or takes an H off of water n Examples – KOH - in drain cleaner – Na. OH - in drain cleaner – NH 3 - ammonia n

Properties of bases Taste bitter n Feel slippery n Can be strong or weak electrolytes n Indicators (turns red litmus paper blue) n React with acids to form water and a salt n

Bases KOH K+ + OH– Strong bases ionize completely and makes lots of ions – Are dangerous NH 3 + H 2 O NH 4+ + OH– Weak acids only make a few ions – Are dangerous if concentrated

How Acidic? More H 3 O+ is more acidic n Measured with p. H n Lower p. H is more acidic n As H 3 O+ goes down, OH- goes up n Higher p. H more basic n p. H of 7 is neutral n 0 7 14 Acidic Neutral Basic

p. H Scale
p. H Low p. H is acid – Lots of H 3 O+ – Little OHn High p. H is base – Little H 3 O+ – Lots of OHn
Neutralization Reactions n A type of double displacement reaction – A reaction b/w an acid and base H 3 O+ + Cl- + Na+ + OH- Cl- + Na+ + 2 H 2 O – Salt and water is always a product of the reaction – Product will be neutral if the right amounts of strong acids and bases are added

Neutralization Reactions As you add acid to a base the p. H drops n As you add base to and acid the p. H rises n

Soap Water and oil don’t mix n Water is polar n Oil is nonpolar n Soap can dissolve in both oil and water n Made by mixing fats with lye (Na. OH) n
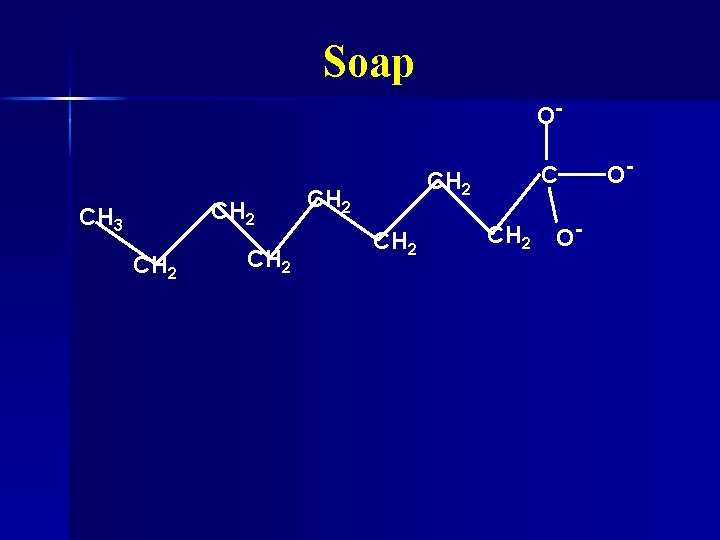
Soap O- CH 2 CH 3 CH 2 CH 2 O- O-
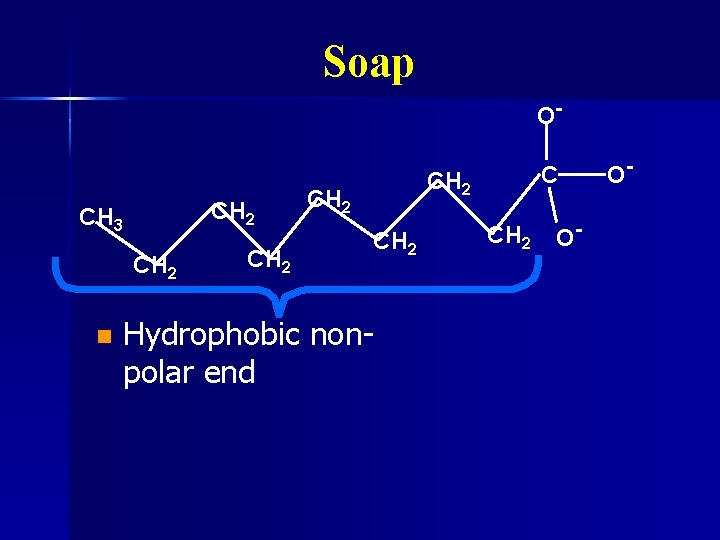
Soap O- CH 2 CH 3 CH 2 n CH 2 CH 2 Hydrophobic nonpolar end CH 2 O- O-
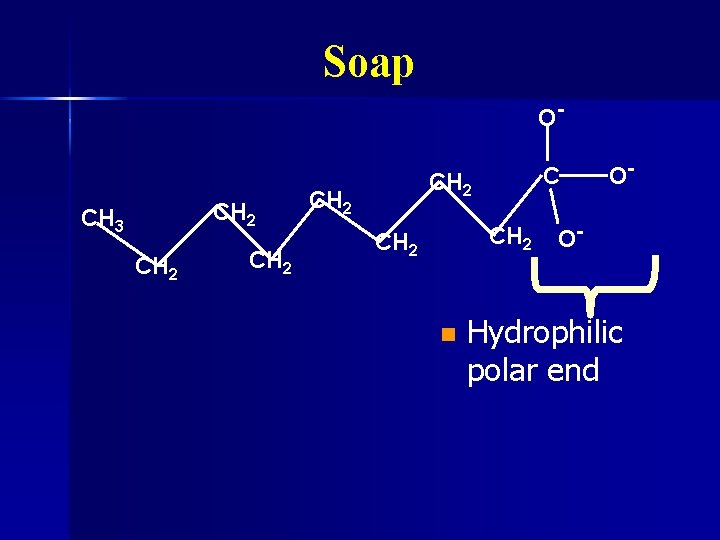
Soap O- CH 2 CH 3 CH 2 CH 2 n O- O- Hydrophilic polar end
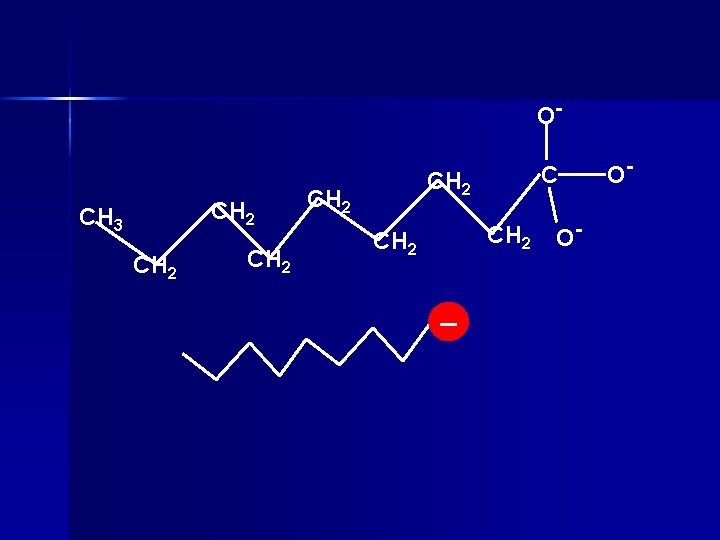
O- CH 2 CH 3 CH 2 CH 2 _ O- O-

A drop of grease in water n Grease is non-polar n Water is polar n Soap lets you dissolve the non-polar in the polar. n
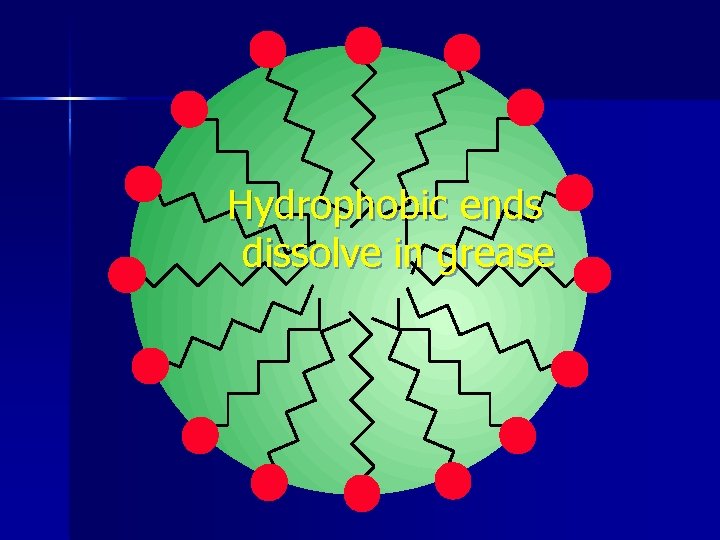
Hydrophobic ends dissolve in grease
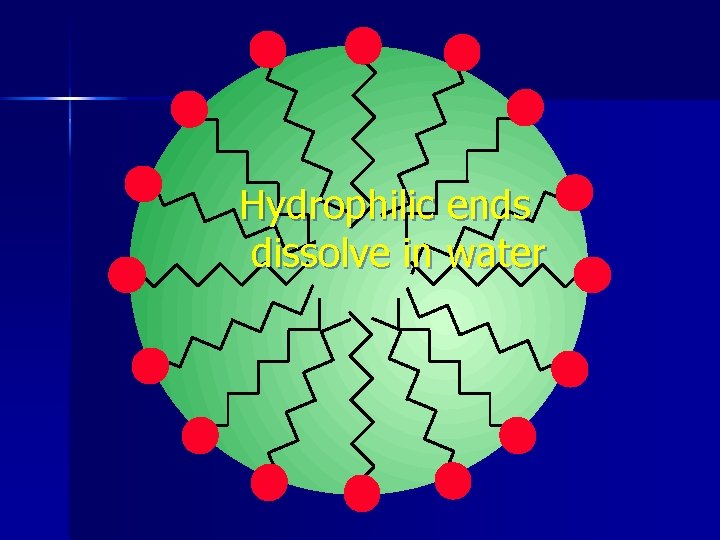
Hydrophilic ends dissolve in water
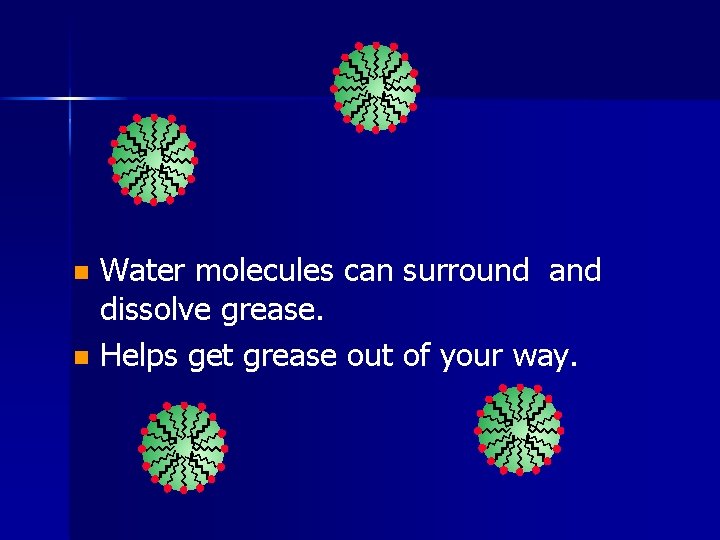
Water molecules can surround and dissolve grease. n Helps get grease out of your way. n

Detergents Soaps react with minerals in hard water and form scum that doesn’t dissolve n Detergents have the same basic structure but have a sulfur at the end, n And start from petroleum n Dissolve in hard water n

Household Uses Antacids- Weak bases that neutralize excess stomach acid n Shampoo- made from detergents n Need to keep p. H between 5 and 8 or it will make the hair dull n Citric acid keeps fruit from browning n Acidic marinades tenderize meats n Drain cleaners are strong bases n
- Slides: 25