PHYSICAL QUANTITY pengukuran Physical Measurement Method Metode Pengukuran

















- Slides: 43

PHYSICAL QUANTITY pengukuran Physical Measurement Method (Metode Pengukuran Fisika) SF 091306 1 Gatut Yudoyono Physics department, MIPA-ITS Physical Quantity Besaran fisika: pengamatan dan

Like all other sciences, physics is based on experimental observations and quantitative measurements. Physical Quantity The main objective of physics is to find the limited number of fundamental laws that govern natural phenomena and to use them to develop theories that can predict the results of future experiments. The fundamental laws used in developing theories are expressed in the language of mathematics, the tool that provides a bridge between theory and experiment. 2

The need for assigning numerical values to various measured physical quantities was expressed by Lord Kelvin (William Thomson) as follows: Physical Quantity “I often say that when you can measure what you are speaking about, and express i t in numbers, numbers you should know something about it, but when you cannot express it in numbers, your knowledge is of a meager and unsatisfactory kind. It may be the beginning of knowledge but you have scarcely in your thoughts advanced to the state of science. ” 3

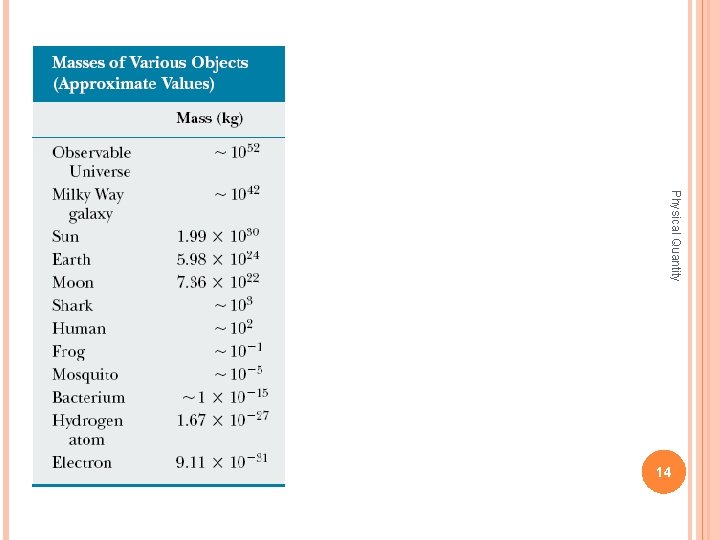
The laws of physics are expressed as mathematical relationships among physical quantities Most of these quantities are derived quantities, Physical Quantity in that they can be expressed as combinations of a small number of basic quantities. In mechanics, the three basic quantities are • Length • Mass • Time. All other quantities in mechanics can be expressed in terms of these three. 4

Standards of Length, Mass, and Time If we are to report the results of a measurement to someone who wishes to reproduce this measurement, a standard must be defined. Physical Quantity It would be meaningless if a visitor from another planet were to talk to us about a length of 8 “glitches” if we do not know the meaning of the unit glitch. Likewise, if we are told that a person has a mass of 75 kilograms and our unit of mass is defined to be 1 kilogram, then that person is 75 times as massive as our basic unit. Whatever is chosen as a standard must be readily accessible and possess some property that can be measured reliably. Measurements taken by different people in different places must yield the same result. 5

A unit is a particular physical quantity, defined and adopted by convention, with which other particular quantities of the same kind are compared to express their value. Physical Quantity The value of a physical quantity is the quantitative expression of a particular physical quantity as the product of a number and a unit, the number being its numerical value. For example, the circumference of the earth around the equator is given by: Ce = 40. 074. 103 m where Ce is the physical quantity and the number 40. 074. 103 is the numerical value of this quantity expressed in the unit "meter". 6

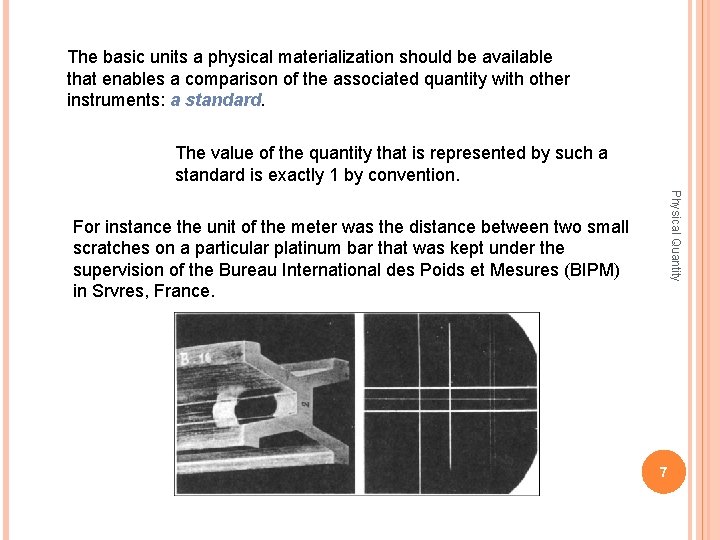
The basic units a physical materialization should be available that enables a comparison of the associated quantity with other instruments: a standard. The value of the quantity that is represented by such a standard is exactly 1 by convention. Physical Quantity For instance the unit of the meter was the distance between two small scratches on a particular platinum bar that was kept under the supervision of the Bureau International des Poids et Mesures (BIPM) in Srvres, France. 7

For other units too (primary) standards have been constructed, and they have been used for a long time to compare the unit value with other standards, called secondary standards. Physical Quantity The unit value for the quantities mass, length and time have been chosen with a view to practical applicability. For instance, the circumference of the earth could be a proper standard but is highly impractical; therefore the meter was originally defined (1791) as one ten millionth of a quarter of the earth's meridian passing through Paris, which is a much more practical measure for daily life usage. 8

Length In A. D. 1120 the king of England decreed that the standard of length in his country would be named the yard (3 ft) and would be precisely equal to the distance from the tip of his nose to the end of his outstretched arm. Physical Quantity Similarly, the original standard for the foot adopted by the French was the length of the royal foot of King Louis XIV (until 1799). The legal standard of length in France became the meter, defined as one ten-millionth the distance from the equator to the North Pole along one particular longitudinal line that passes through Paris. 9

Evidently, these physical standards were much more stable and reproducible than those defined earlier on the basis of human properties. Physical Quantity Length of a foot, defined as the average of the feet of 12 men 10

As recently as 1960, the length of the meter was defined as the distance between two lines on a specific platinum–iridium bar stored under controlled conditions in France. 1 650 763. 73 wavelengths of orange-red light emitted from a krypton-86 lamp. Physical Quantity In the 1960 s and 1970 s, the meter was defined as However, in October 1983, the meter (m) was redefined as the distance traveled by light in vacuum during a time of 1/299 792458 second. 11

Physical Quantity 12

Mass The SI unit of mass, the kilogram (kg), is defined as the mass of a specific platinum–iridium alloy cylinder kept at the International Bureau of Weights and Measures at Sèvres, France. A duplicate of the Sèvres cylinder is kept at the National Institute of Standards and Technology (NIST) in Gaithersburg, Maryland Physical Quantity This mass standard was established in 1887 and has not been changed since that time because platinum–iridium is an unusually stable alloy. 13

Physical Quantity 14

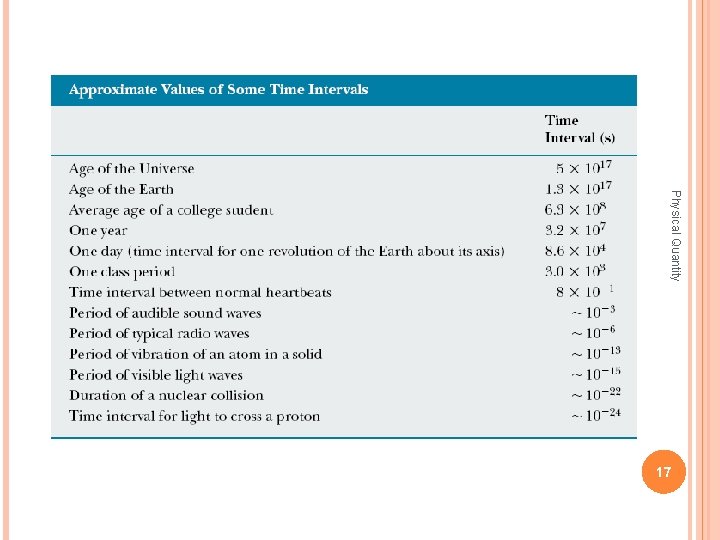
Time Before 1960, the standard of time was defined in terms of the mean solar day for the year 1900. The second was defined as Physical Quantity (A solar day is the time interval between successive appearances of the Sun at the highest point it reaches in the sky each day. ) of a mean solar day. The rotation of the Earth is now known to vary slightly with time, however, and therefore this motion is not a good one to use for defining a time standard. 15

In 1967, the second was redefined to take advantage of the high precision attainable in a device known as an atomic clock , which uses the characteristic frequency of the cesium-133 atom as the “reference clock. ” Physical Quantity The second (s) is now defined as 9 192 631770 times the period of vibration of radiation from the cesium atom. 16

Physical Quantity 17

Physical Quantity 18

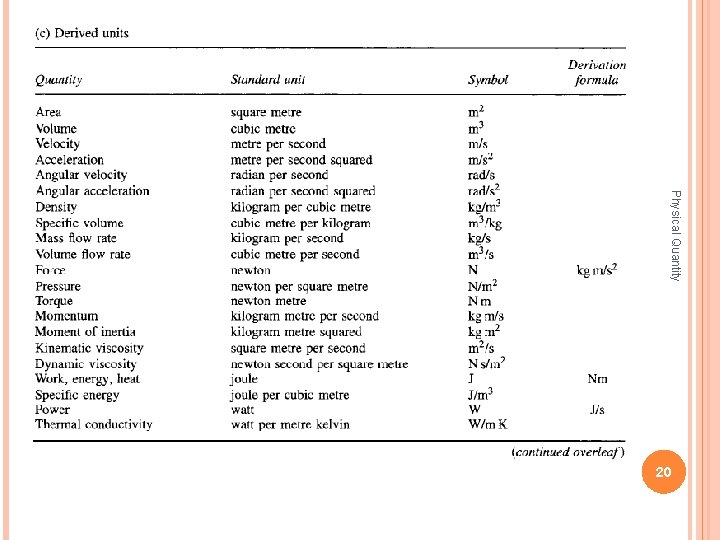
Physical Quantity 19

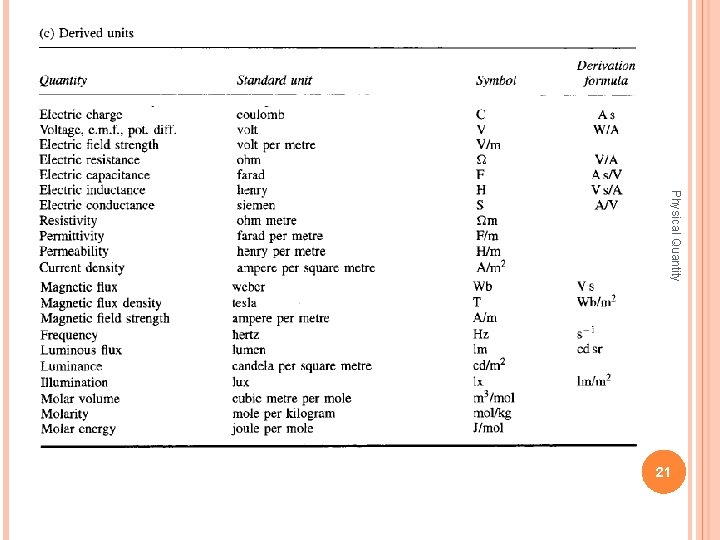
Physical Quantity 20

Physical Quantity 21

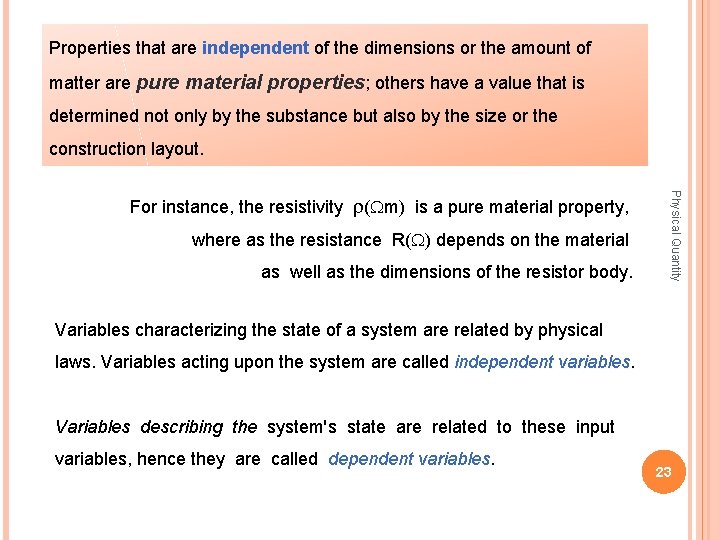
Quantities and properties A quantity is a property ascribed to phenomena, processes or objects, to which a value or a class can be assigned. Quantities can be classified in many ways into groups. a quantity with magnitude and direction is called a vector quantity. Physical Quantity Quantities that have magnitude only are called scalar quantities; We distinguish energy related quantities (associated with energetic phenomena, for instance force, electric current) and quantities that are not associated with energy (static quantities or simply properties, for instance resistivity, length). 22

Properties that are independent of the dimensions or the amount of matter are pure material properties; others have a value that is determined not only by the substance but also by the size or the construction layout. where as the resistance R(W) depends on the material as well as the dimensions of the resistor body. Physical Quantity For instance, the resistivity r(Wm) is a pure material property, Variables characterizing the state of a system are related by physical laws. Variables acting upon the system are called independent variables. Variables describing the system's state are related to these input variables, hence they are called dependent variables 23

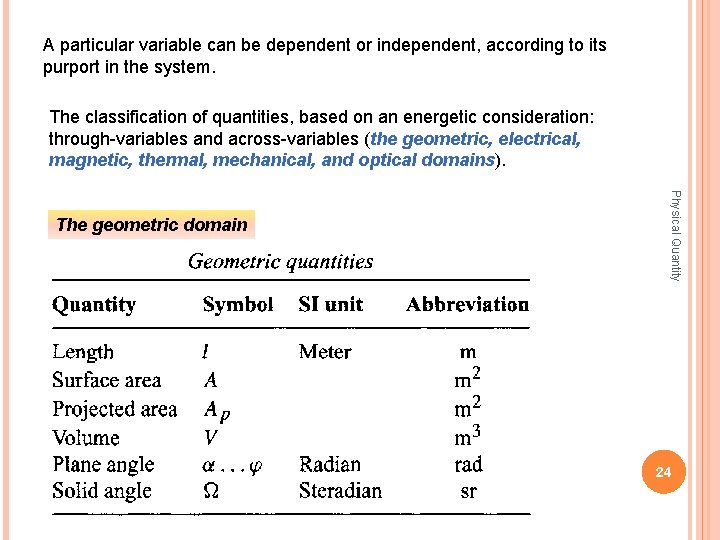
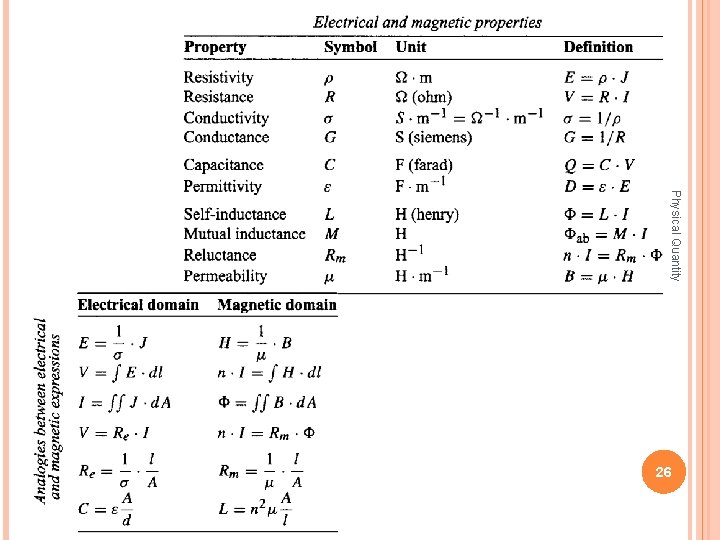
A particular variable can be dependent or independent, according to its purport in the system. The classification of quantities, based on an energetic consideration: through-variables and across-variables (the geometric, electrical, magnetic, thermal, mechanical, and optical domains). domains Physical Quantity The geometric domain 24

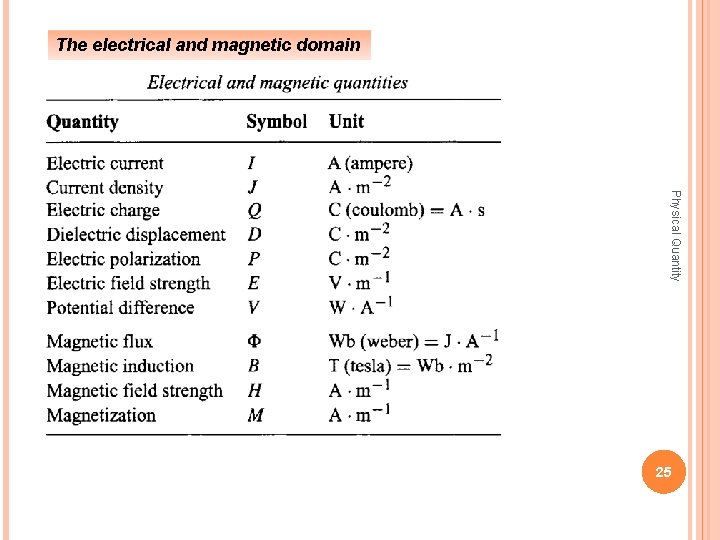
The electrical and magnetic domain Physical Quantity 25

Physical Quantity 26

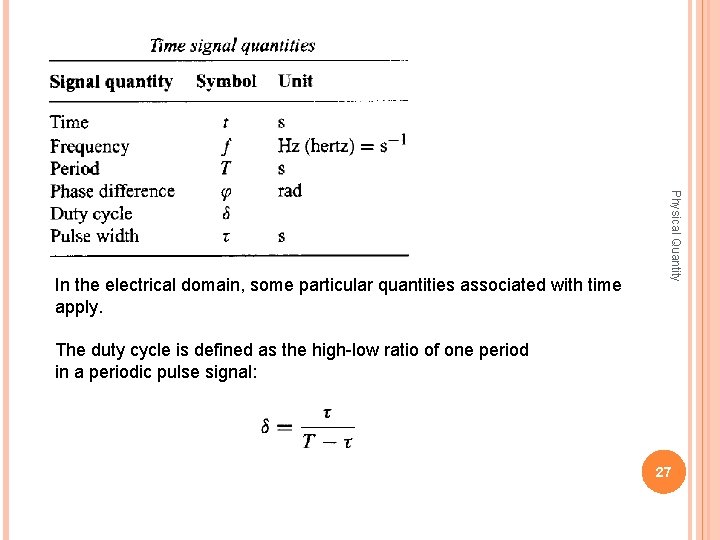
Physical Quantity In the electrical domain, some particular quantities associated with time apply. The duty cycle is defined as the high-low ratio of one period in a periodic pulse signal: 27

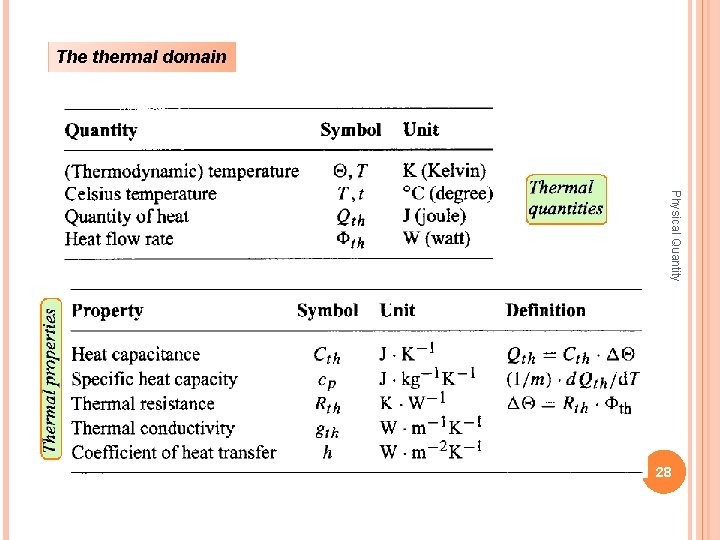
The thermal domain Physical Quantity 28

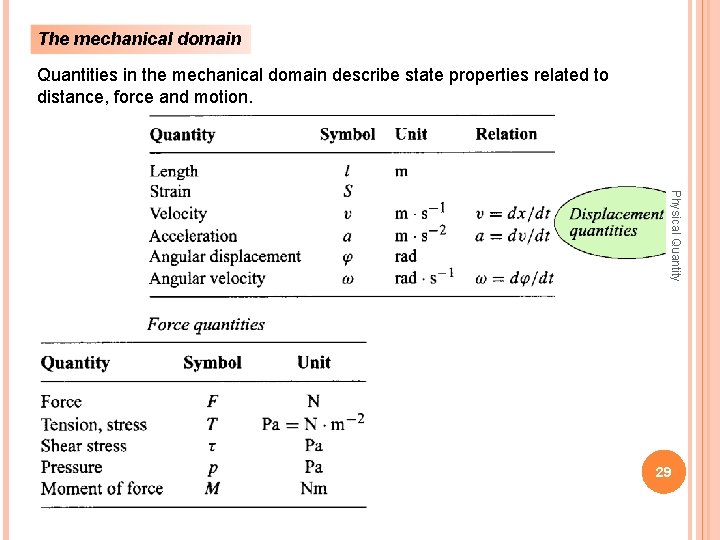
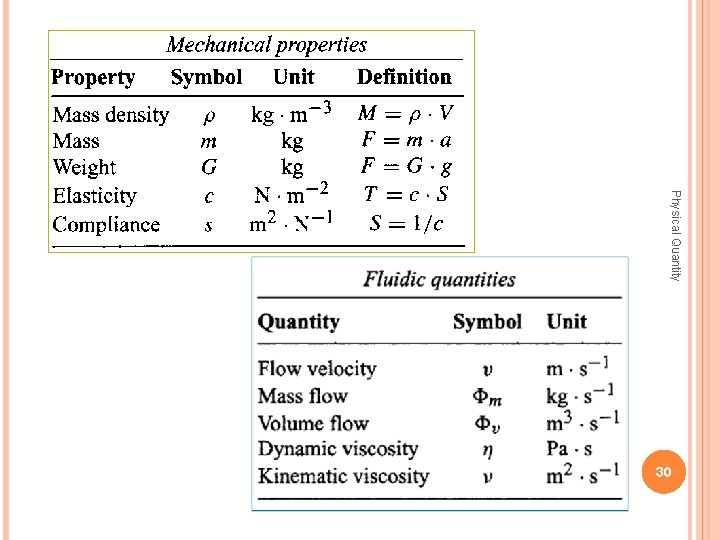
The mechanical domain Quantities in the mechanical domain describe state properties related to distance, force and motion. Physical Quantity 29

Physical Quantity 30

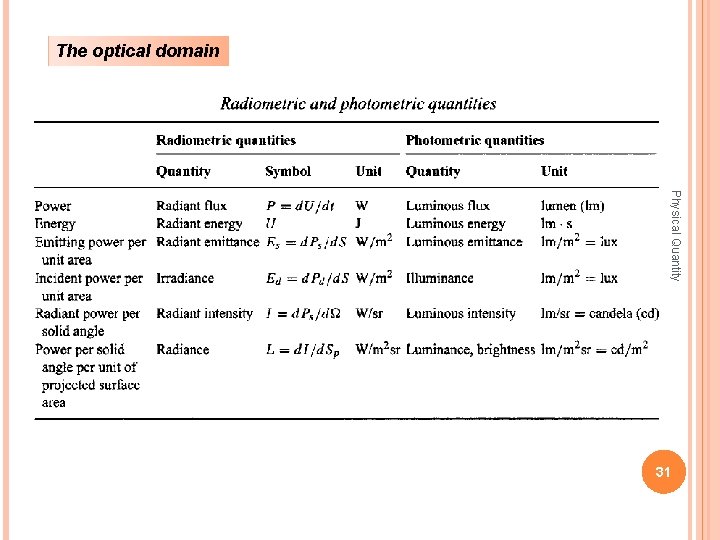
The optical domain Physical Quantity 31

Physical Quantity 32

Physical Quantity 33

Physical Quantity 34

Physical Quantity e d n 35

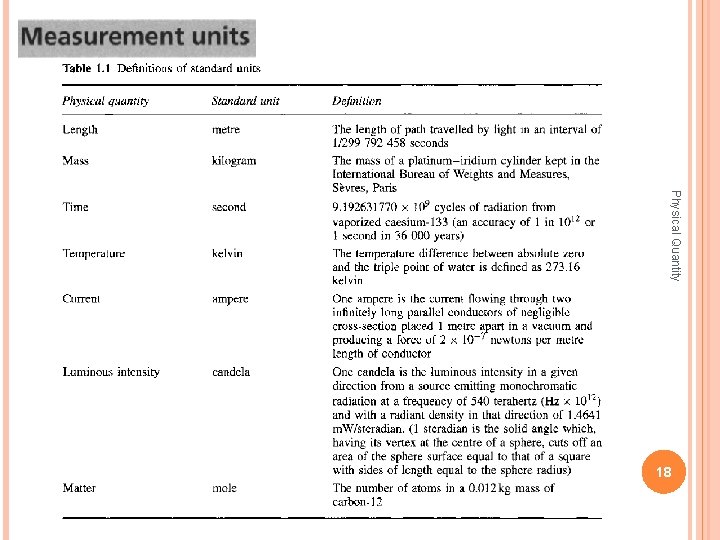
The seven standard units are defined as follows: The meter is the length of the path travelled by light in vacuum during a time interval of 1/299 792 458 of a second [17 th CGPM (1983), Res. 1]. Physical Quantity A former standard meter, " left: end view of the British copy of the International Meter; right: rulings on the polished facet; the two thick vertical lines indicate the length at 0~ and 20~ taking into account thermal expansion [National Physical Laboratory, Teddington, Middlesex] CGPM = Conference Generale des Poids et Mesures 36

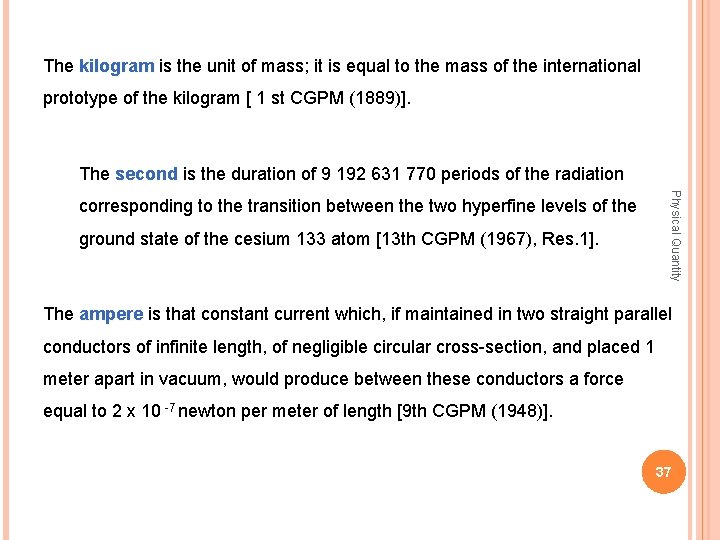
The kilogram is the unit of mass; it is equal to the mass of the international prototype of the kilogram [ 1 st CGPM (1889)]. The second is the duration of 9 192 631 770 periods of the radiation ground state of the cesium 133 atom [13 th CGPM (1967), Res. 1]. Physical Quantity corresponding to the transition between the two hyperfine levels of the The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 meter apart in vacuum, would produce between these conductors a force equal to 2 x 10 -7 newton per meter of length [9 th CGPM (1948)]. 37

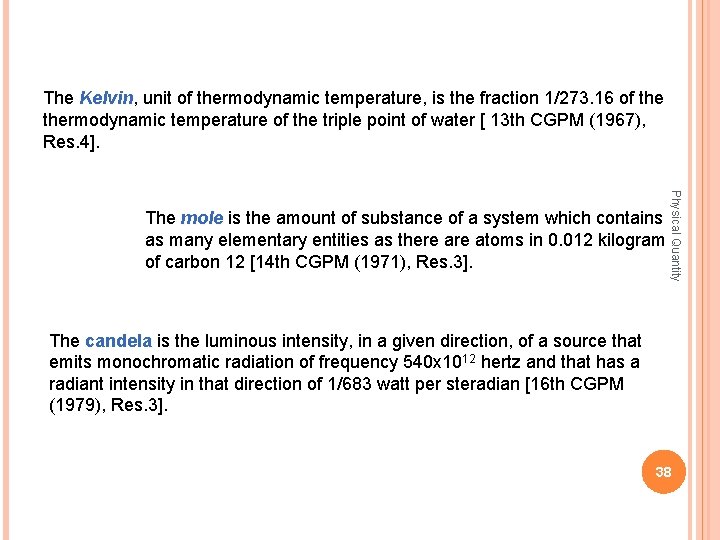
The Kelvin, Kelvin unit of thermodynamic temperature, is the fraction 1/273. 16 of thermodynamic temperature of the triple point of water [ 13 th CGPM (1967), Res. 4]. Physical Quantity The mole is the amount of substance of a system which contains as many elementary entities as there atoms in 0. 012 kilogram of carbon 12 [14 th CGPM (1971), Res. 3]. The candela is the luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency 540 x 1012 hertz and that has a radiant intensity in that direction of 1/683 watt per steradian [16 th CGPM (1979), Res. 3]. 38

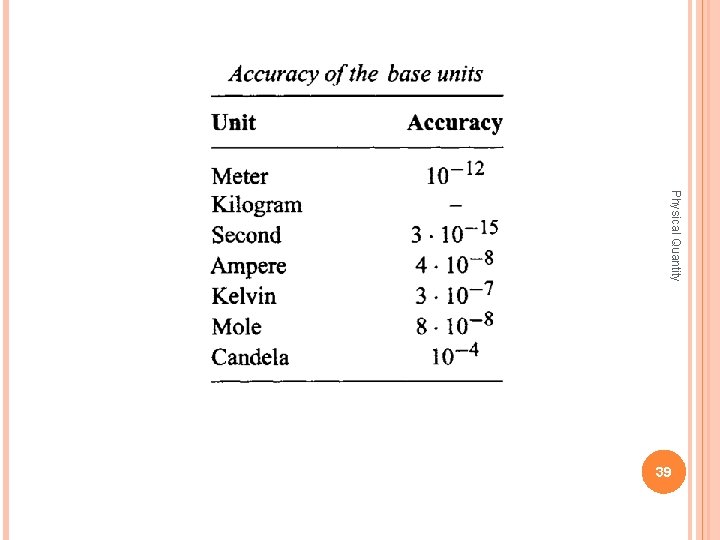
Physical Quantity 39

Physical quantity is the numerical value of a measurable property that describes a physical system's state at a moment in time. which describes a physical system's state at any given moment in time. Physical Quantity A physical property is any measurable property the value of For that reason the changes in the physical quantities of a system describe its transformation (or evolution between its momentary states). 40

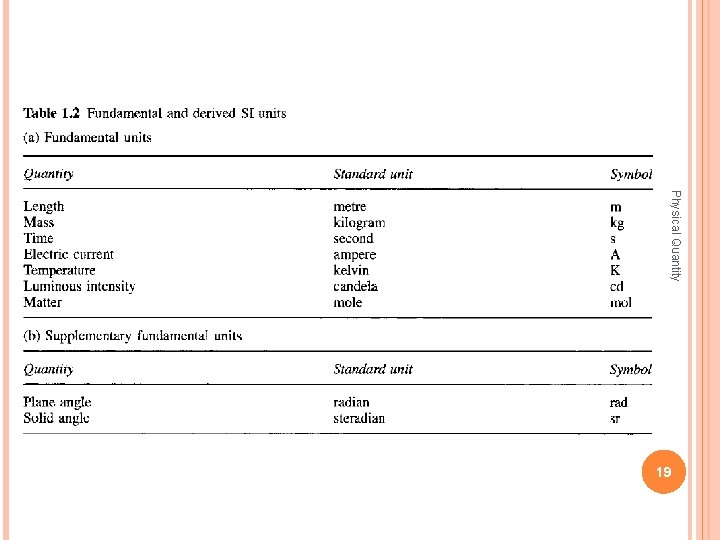
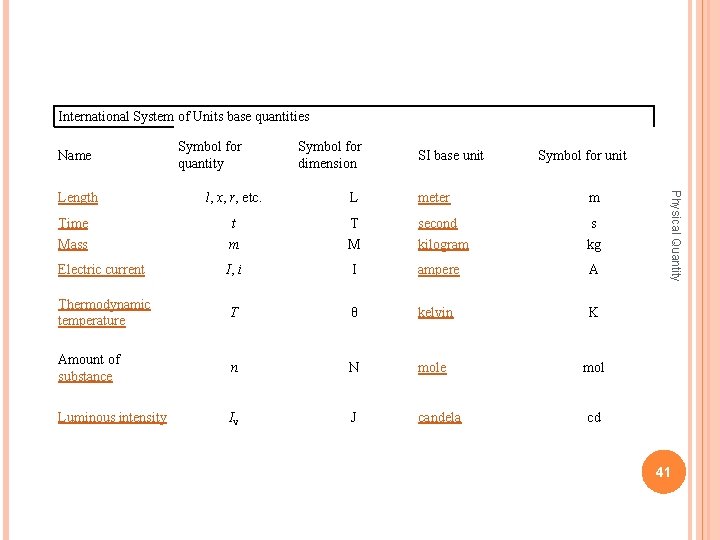
International System of Units base quantities Name Symbol for dimension SI base unit Symbol for unit l, x, r, etc. L meter m Time t T second s Mass m M kilogram kg Electric current I, i I ampere A Thermodynamic temperature T θ kelvin K Amount of substance n N mole mol Luminous intensity Iv J candela Physical Quantity Length Symbol for quantity cd 41

The physical properties of an object are defined traditionally in a Newtonian sense; the physical properties of an object may include, but are not limited to: • electric charge • electric field • electric potential • emission • flow rate • fluidity • frequency • impedance • inductance • intensity • irradiance • length • location • luminance • luster • malleability • magnetic field • magnetic flux • mass • melting point • momentum • permeability • permittivity • pressure • radiance • solubility • specific heat • resistance • reflectivity • spin • strength • temperature • tension • thermal transfer • velocity • viscosity • volume Physical Quantity • absorption • albedo • area • brittleness • boiling point • capacitance • color • concentration • conductance • density • dielectric • ductility • distribution • efficacy 42

A quantity is called: • extensive when its magnitude is additive for subsystems (volume, mass, etc. ) • intensive when the magnitude is independent of the extent of the system (temperature, pressure, etc. ) Physical Quantity There also physical quantities that can be classified as neither extensive nor intensive, for example angular momentum, area, force, length, and time. 43