Physical Quantities Units Physical Quantities and Units refer



- Slides: 10

Physical Quantities & Units Physical Quantities and Units refer to measurement. Measurement involves counting, but we can only count numbers 1, 2, 3, 4. . . of a particular item. - The other thing we can do is to count the number of times a physical quantity is greater or less for one case than in another case. - A complete specification of the method of counting specifies a unit.

How units are defined - A unit has to have a special name and symbol. The usefulness of a unit is as a means of communicating to everyone who does science how it was particularly defined. - To make a desk of a certain length, breath and height it is necessary to determine ratio of its dimensions as compared to the defined unit. ie. A numerical magnitude x its unit Therefore, defined units must be: - unambiguously defined - reproducible to a great accuracy - accepted by the most people

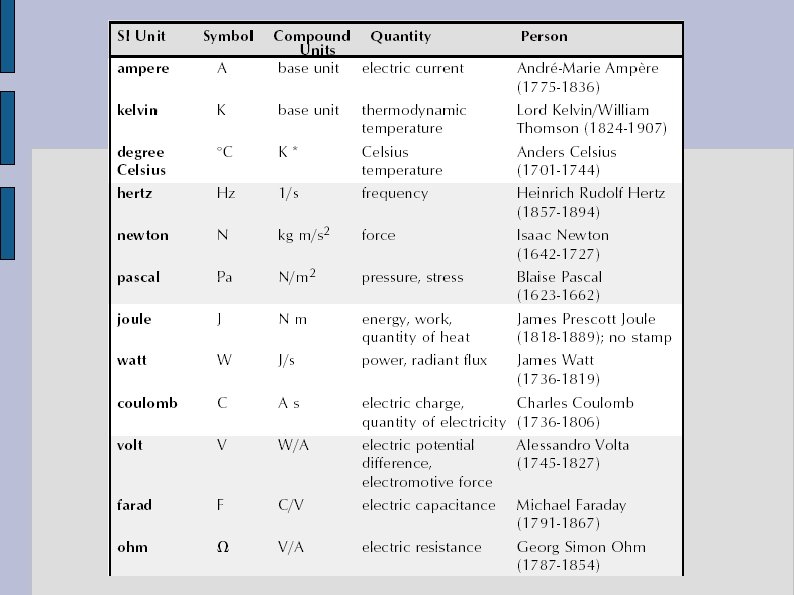
SI units - The SI units (adopted in 1960) represent a set of basic physical quantities. - Second(s). The second is defined as a number of the period of vibration of radiation from the cesium -133 atom. - Metre (m). The distance travelled by light in vacuum during a time of 1/299 792 458 second. - Kilogramme (kg). Defined as the mass of a specific platinum-iridium alloy cylinder kept at the International Bureau of Weights and Measures in France.

SI Units - Kelvin (K). The kelvin is defined as 1/273. 16 of the temperature of the triple point of water. - Ampere (A). Defined as the constant current which, if maintained in two straight parallel conductors of infinite length, of negligible cross section, and placed one metre apart in vacuum, would provide between the conductors a force equal to 2 x 10 -7 newton per metre of length. - Mole (mol). The amount of substance containing as many identical units as there atoms in 12 grams of carbon-12.


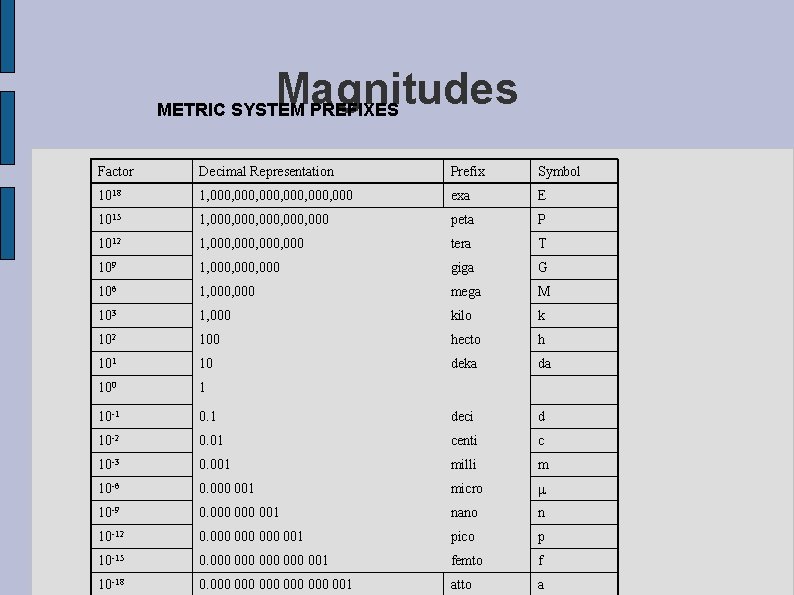
Magnitudes METRIC SYSTEM PREFIXES Factor Decimal Representation Prefix Symbol 1018 1, 000, 000 exa E 1015 1, 000, 000 peta P 1012 1, 000, 000 tera T 109 1, 000, 000 giga G 106 1, 000 mega M 103 1, 000 kilo k 102 100 hecto h 101 10 deka da 100 1 10 -1 0. 1 deci d 10 -2 0. 01 centi c 10 -3 0. 001 milli m 10 -6 0. 000 001 micro m 10 -9 0. 000 001 nano n 10 -12 0. 000 000 001 pico p 10 -15 0. 000 000 001 femto f 10 -18 0. 000 000 000 001 atto a

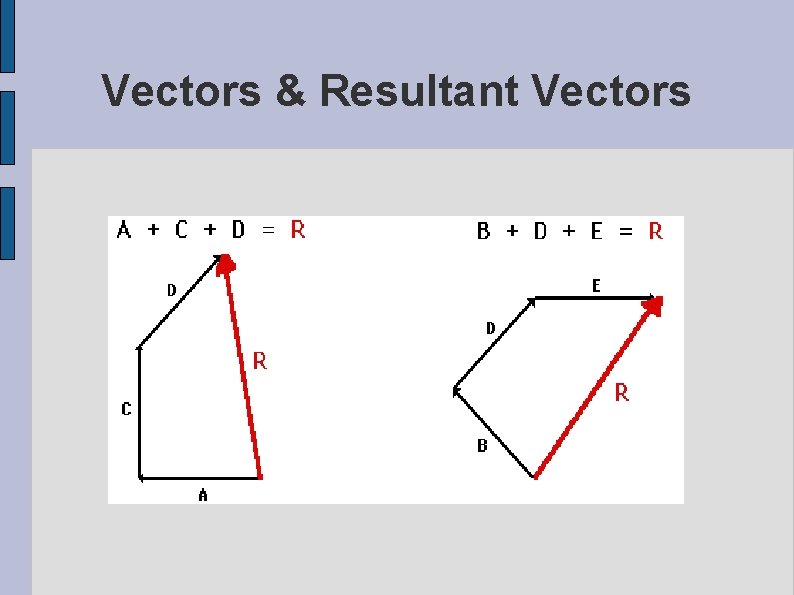
Scalars and Vectors Scalars are quantities which are fully described by a magnitude alone. Examples are all the SI base units which are all described by their numerical magnitudes. Vectors are quantities which are fully described by both a magnitude and a direction.

Vectors & Resultant Vectors

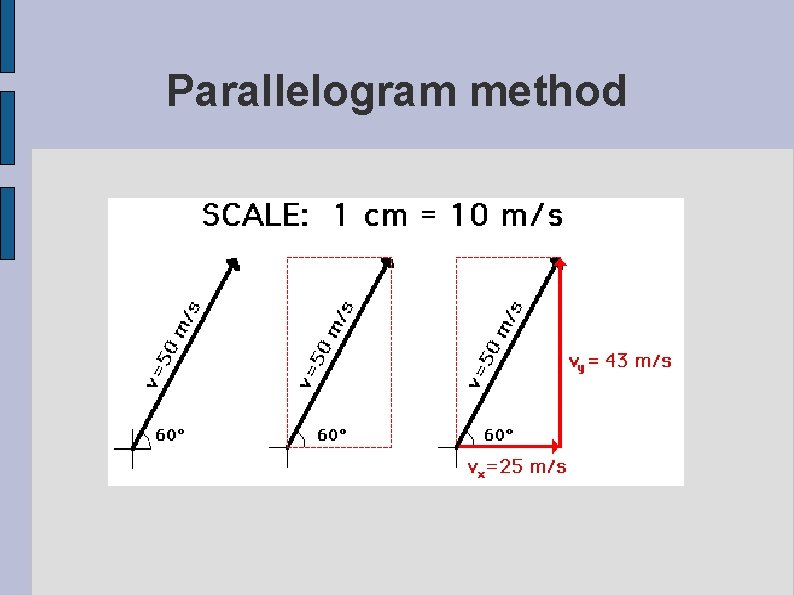
Parallelogram method
