Physical Properties of Water Water statistics why understanding





- Slides: 8

Physical Properties of Water

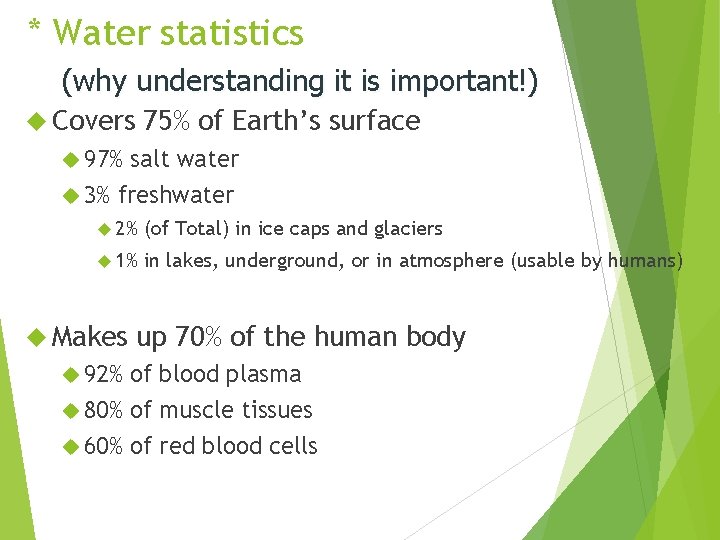
* Water statistics (why understanding it is important!) Covers 75% of Earth’s surface 97% salt water 3% freshwater 2% (of Total) in ice caps and glaciers 1% in lakes, underground, or in atmosphere (usable by humans) Makes 92% up 70% of the human body of blood plasma 80% of muscle tissues 60% of red blood cells

Physical properties Water: Is clear, colorless, odorless, and tasteless * Colors, tastes and odors are caused by substances dissolved in the water. Boils at 100°C (212 F) Freezes at 0°C (32 F) Density = 1. 0 g/m. L (at 4°C) Water is a Polar Molecule

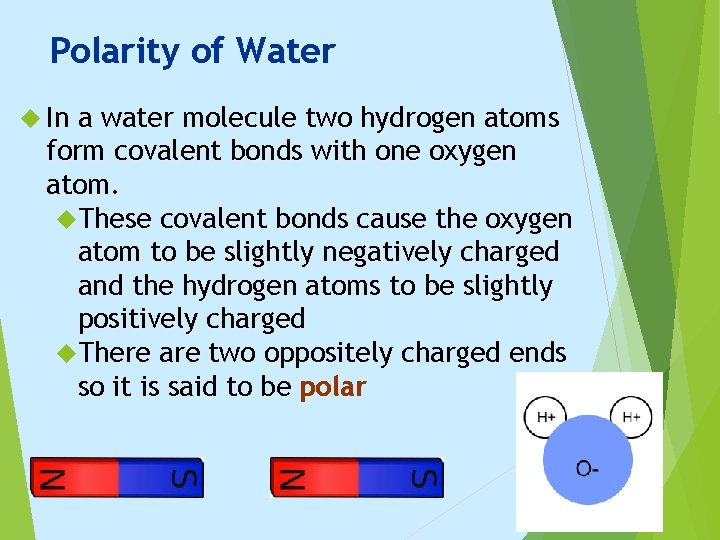
Polarity of Water In a water molecule two hydrogen atoms form covalent bonds with one oxygen atom. These covalent bonds cause the oxygen atom to be slightly negatively charged and the hydrogen atoms to be slightly positively charged There are two oppositely charged ends so it is said to be polar

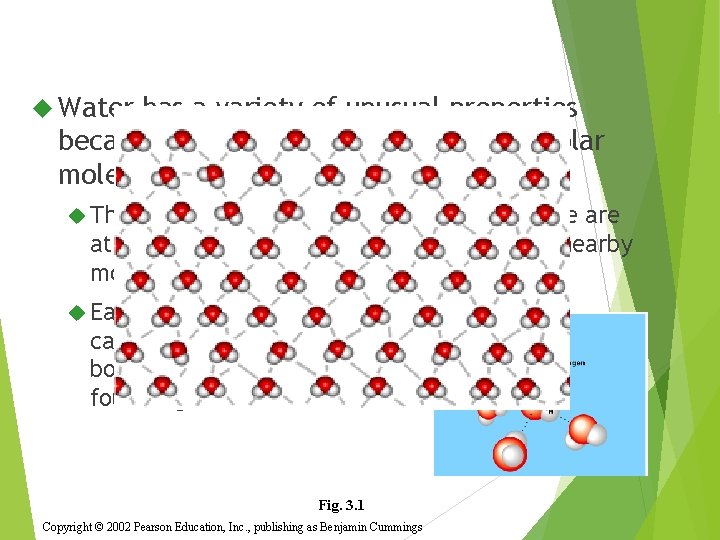
Water has a variety of unusual properties because of attractions between these polar molecules. The slightly negative regions of one molecule are attracted to the slightly positive regions of nearby molecules, forming a hydrogen bond. Each water molecule can form hydrogen bonds with up to four neighbors. Fig. 3. 1 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Adhesion – water sticking to other things Ø The adhesion of water leads to two important properties: Water as a “universal” solvent 1. Water only dissolves polar molecules Ex: Salt Sugar Carbon Dioxide Oxygen Capillary action 2. Water will make hydrogen bonds with other surfaces such as glass, soil, plant tissues, and cotton. a) 1. b) Ø Making it able to “climb” objects against the forces of gravity Check this out Adhesion is also what causes water to bead on plants and other materials

Cohesion – water molecules sticking to other water molecules video The cohesion of water molecules leads to two important properties: 1. Surface Tension – the attraction between water molecules at the surface of the liquid a) Water has a greater surface tension than most other liquids because hydrogen bonds among surface water molecules resist stretching or breaking the surface b) Water behaves as if covered by an invisible film. High Specific Heat – it takes a lot of energy to warm up water and it takes a very long time for warm water to cool off 2. 1. Due to the fact that its hydrogen bonds are constantly breaking and reforming Video of water properties

“Water vapor forms a kind of global ‘blanket’ which helps to keep the earth warm. Heat radiated from the sun-warmed surface of the earth is absorbed and held by the vapor. ” http: //www. ec. gc. ca/water/index. htm