Physical Properties of Solutions Chapter 12 Copyright The












- Slides: 38

Physical Properties of Solutions Chapter 12 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

12. 1 - Types of solutions A solution is a homogenous mixture of 2 or more substances The solute is(are) the substance(s) present in the smaller amount(s) The solvent is the substance present in the larger amount 12. 1

A saturated solution contains the maximum amount of a solute that will dissolve in a given solvent at a specific temperature. An unsaturated solution contains less solute than the solvent has the capacity to dissolve at a specific temperature. A supersaturated solution contains more solute than is present in a saturated solution at a specific temperature. Sodium acetate crystals rapidly form when a seed crystal is added to a supersaturated solution of sodium acetate. 12. 1

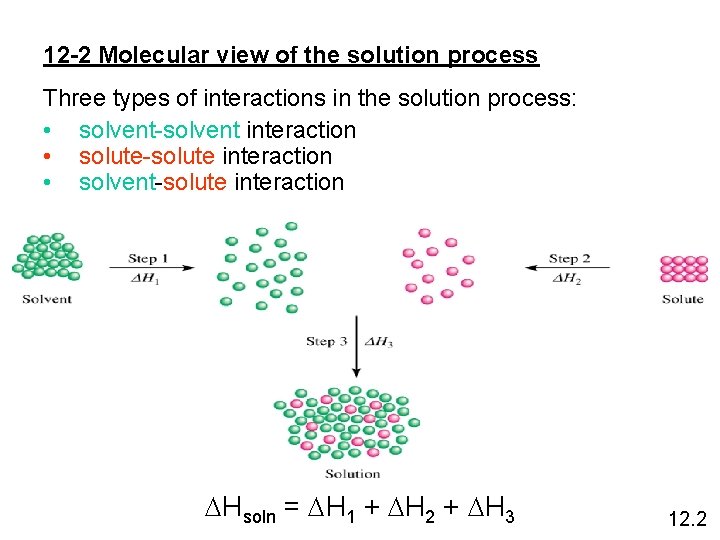
12 -2 Molecular view of the solution process Three types of interactions in the solution process: • solvent-solvent interaction • solute-solute interaction • solvent-solute interaction DHsoln = DH 1 + DH 2 + DH 3 12. 2

“like dissolves like” Two substances with similar intermolecular forces are likely to be soluble in each other. • non-polar molecules are soluble in non-polar solvents CCl 4 in C 6 H 6 • polar molecules are soluble in polar solvents C 2 H 5 OH in H 2 O • ionic compounds are more soluble in polar solvents Na. Cl in H 2 O or NH 3 (l) 12. 2

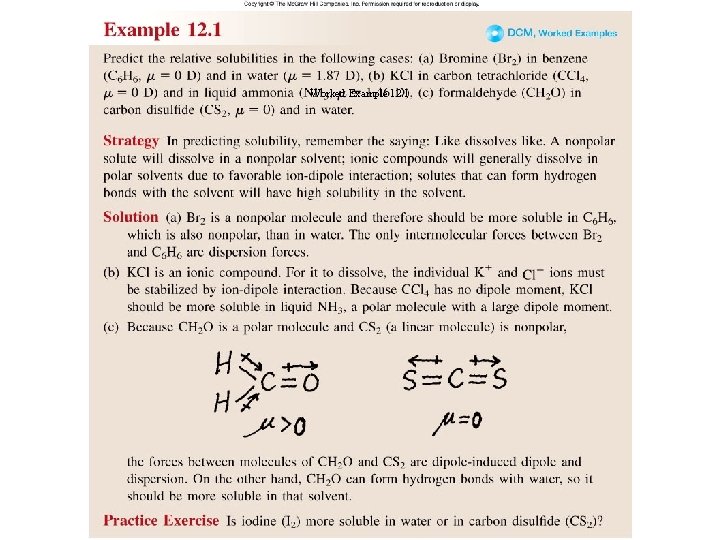
Worked Example 12. 1

12 -3 Concentration Units The concentration of a solution is the amount of solute present in a given quantity of solvent or solution. Percent by Mass mass of solute x 100% % by mass = mass of solute + mass of solvent mass of solute x 100% = mass of solution Mole Fraction (X) moles of A XA = sum of moles of all components 12. 3

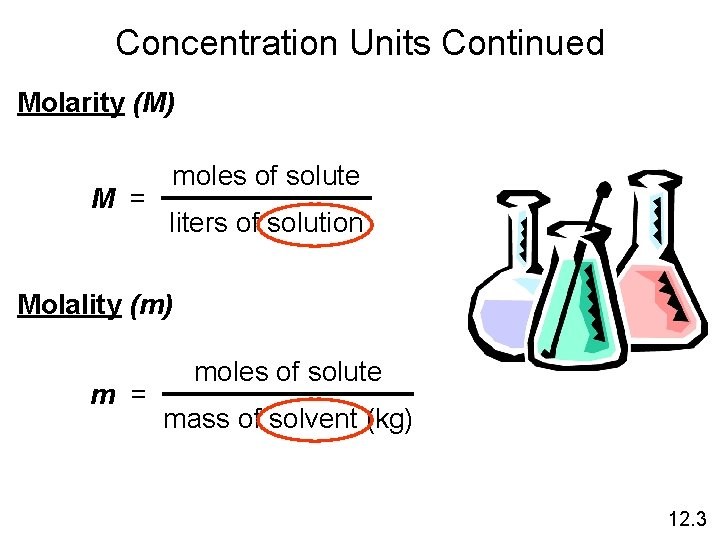
Concentration Units Continued Molarity (M) M = moles of solute liters of solution Molality (m) m = moles of solute mass of solvent (kg) 12. 3

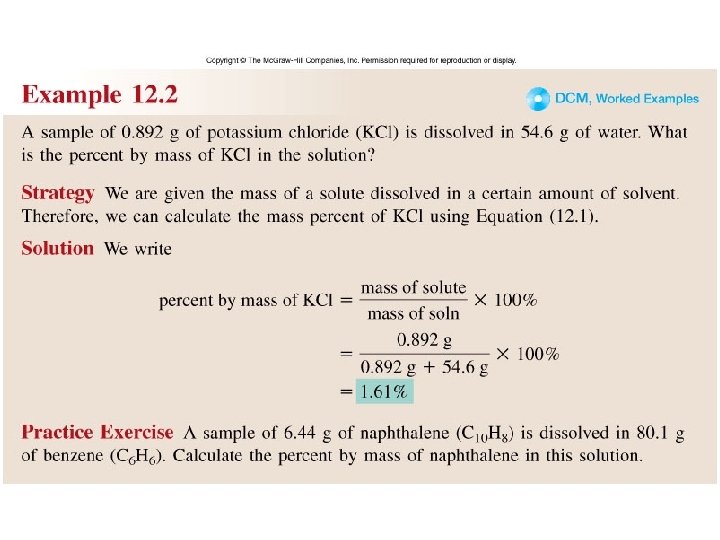
Worked Example 12. 2


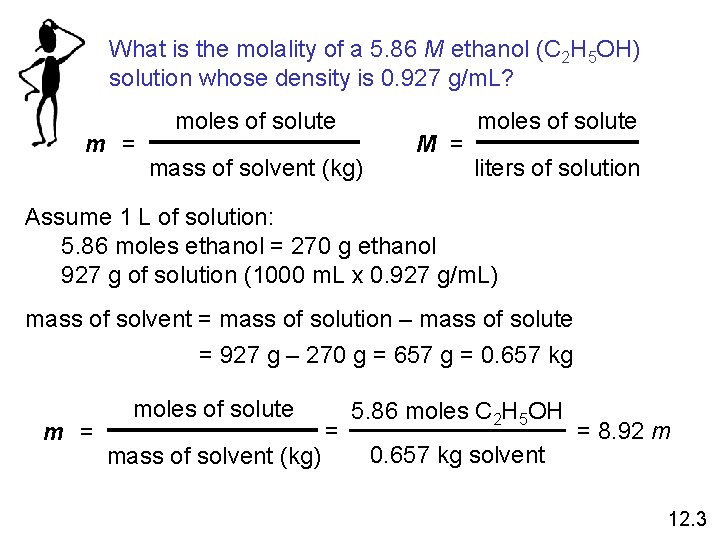
What is the molality of a 5. 86 M ethanol (C 2 H 5 OH) solution whose density is 0. 927 g/m. L? m = moles of solute mass of solvent (kg) M = moles of solute liters of solution Assume 1 L of solution: 5. 86 moles ethanol = 270 g ethanol 927 g of solution (1000 m. L x 0. 927 g/m. L) mass of solvent = mass of solution – mass of solute = 927 g – 270 g = 657 g = 0. 657 kg m = moles of solute mass of solvent (kg) = 5. 86 moles C 2 H 5 OH 0. 657 kg solvent = 8. 92 m 12. 3


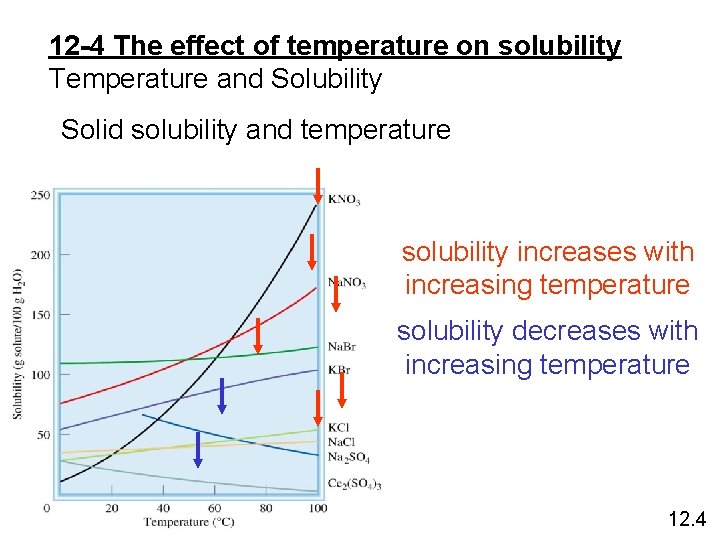
12 -4 The effect of temperature on solubility Temperature and Solubility Solid solubility and temperature solubility increases with increasing temperature solubility decreases with increasing temperature 12. 4

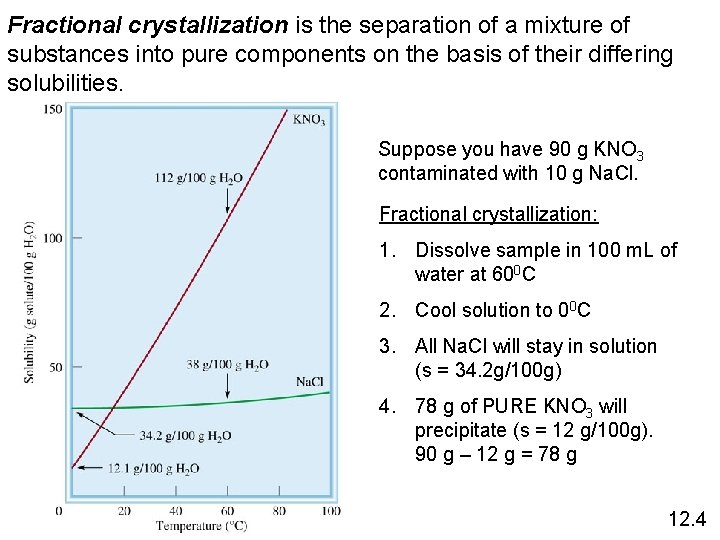
Fractional crystallization is the separation of a mixture of substances into pure components on the basis of their differing solubilities. Suppose you have 90 g KNO 3 contaminated with 10 g Na. Cl. Fractional crystallization: 1. Dissolve sample in 100 m. L of water at 600 C 2. Cool solution to 00 C 3. All Na. Cl will stay in solution (s = 34. 2 g/100 g) 4. 78 g of PURE KNO 3 will precipitate (s = 12 g/100 g). 90 g – 12 g = 78 g 12. 4

Gas Solubility and temperature O 2 gas solubility and temperature solubility usually decreases with increasing temperature 12. 4

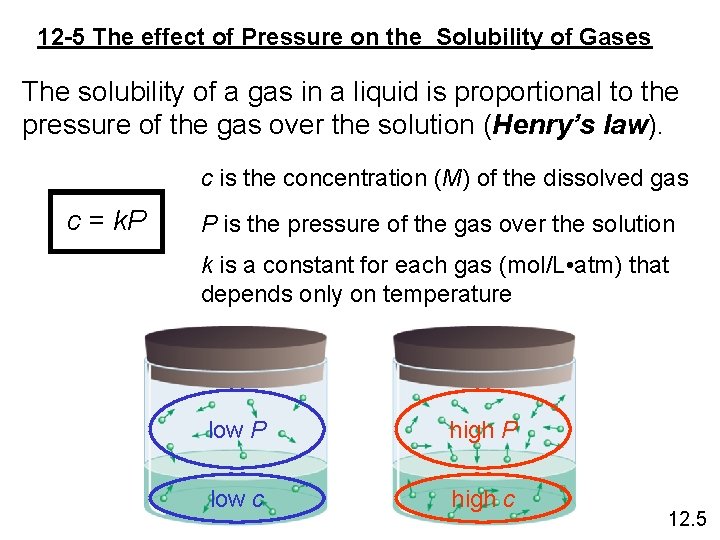
12 -5 The effect of Pressure on the Solubility of Gases The solubility of a gas in a liquid is proportional to the pressure of the gas over the solution (Henry’s law). c is the concentration (M) of the dissolved gas c = k. P P is the pressure of the gas over the solution k is a constant for each gas (mol/L • atm) that depends only on temperature low P high P low c high c 12. 5

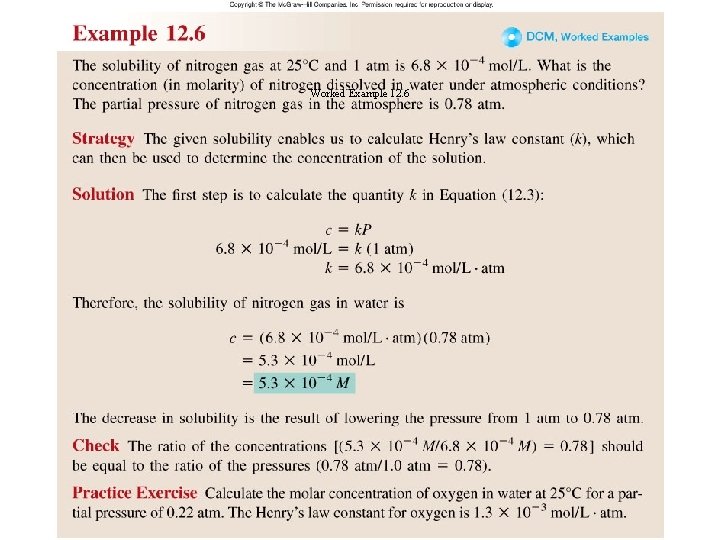
Worked Example 12. 6

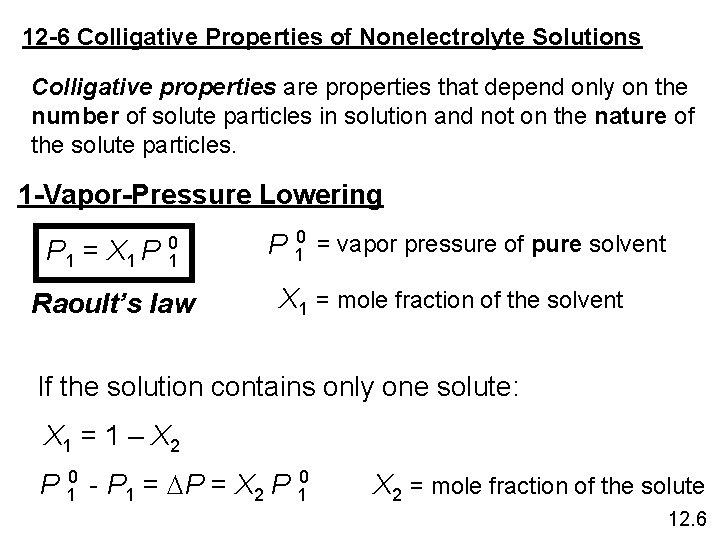
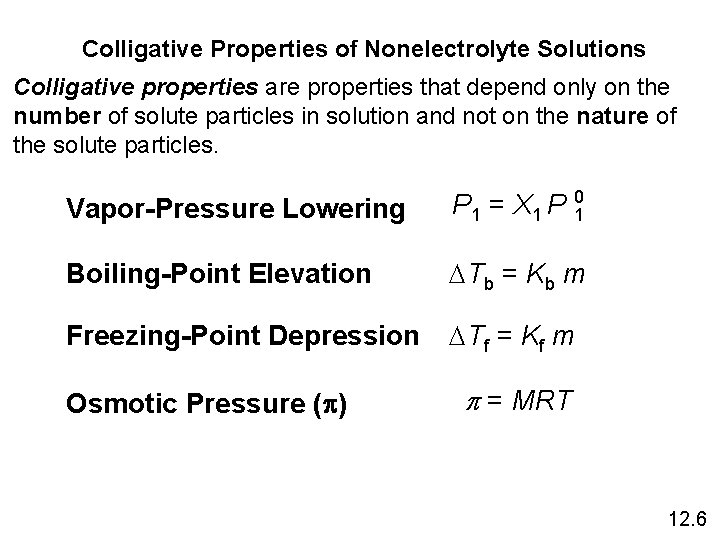
12 -6 Colligative Properties of Nonelectrolyte Solutions Colligative properties are properties that depend only on the number of solute particles in solution and not on the nature of the solute particles. 1 -Vapor-Pressure Lowering P 1 = X 1 P 0 1 Raoult’s law P 10 = vapor pressure of pure solvent X 1 = mole fraction of the solvent If the solution contains only one solute: X 1 = 1 – X 2 P 10 - P 1 = DP = X 2 P 10 X 2 = mole fraction of the solute 12. 6

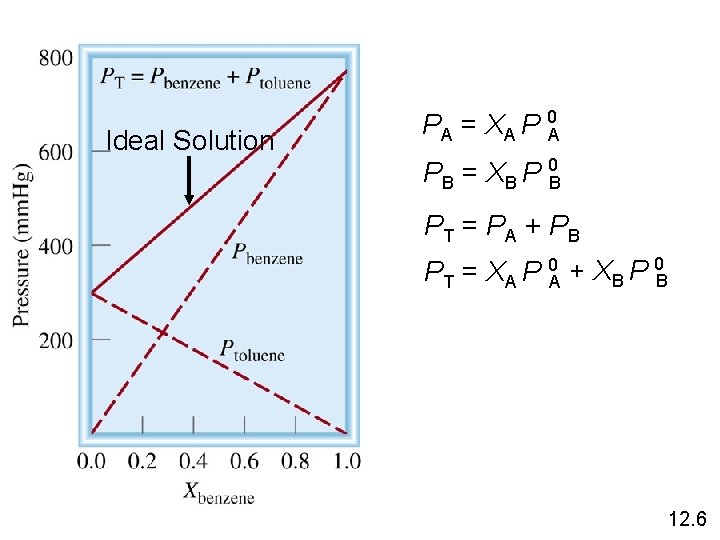
Ideal Solution PA = XA P 0 A PB = XB P 0 B PT = PA + PB PT = XA P 0 A + XB P 0 B 12. 6

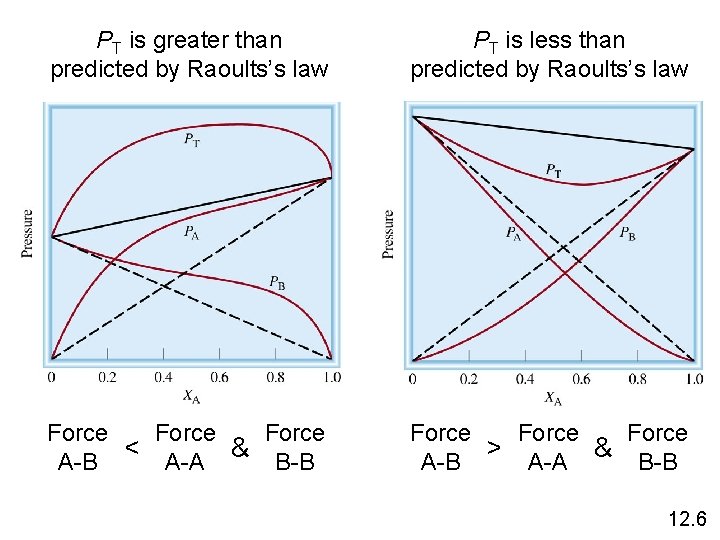
PT is greater than predicted by Raoults’s law PT is less than predicted by Raoults’s law Force < A-A & B-B A-B Force > A-A & B-B A-B 12. 6

Worked Example 12. 7

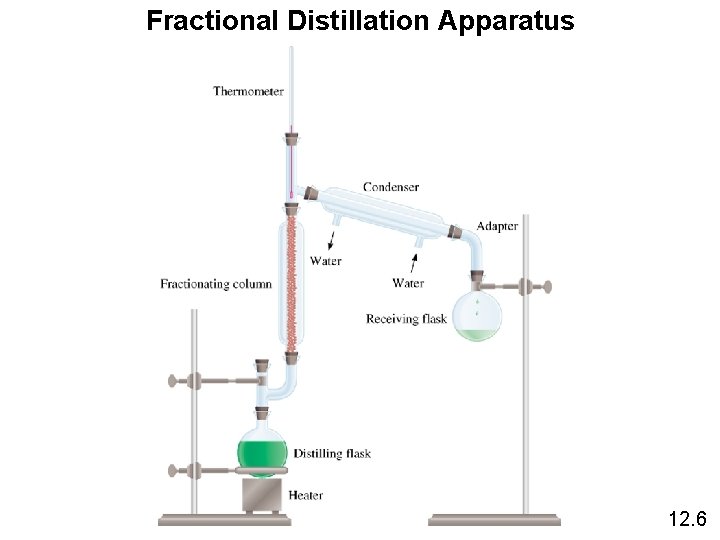
Fractional Distillation Apparatus 12. 6

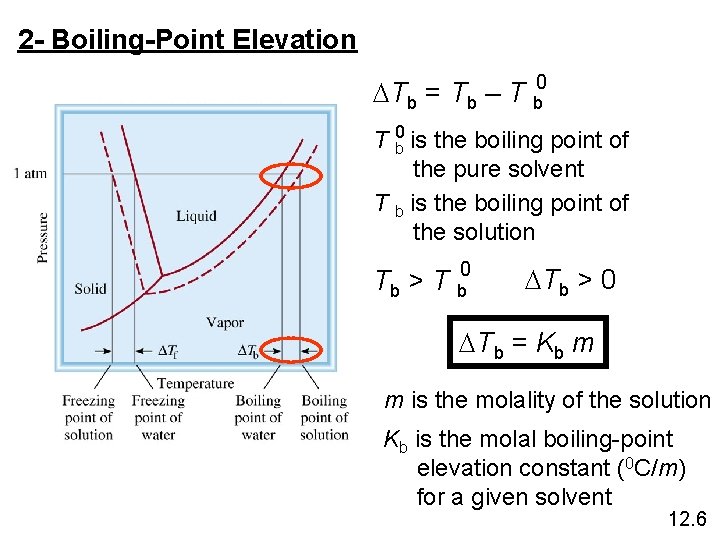
2 - Boiling-Point Elevation DTb = Tb – T b 0 is the boiling point of the pure solvent T b is the boiling point of the solution Tb > T b 0 DTb > 0 DTb = Kb m m is the molality of the solution Kb is the molal boiling-point elevation constant (0 C/m) for a given solvent 12. 6

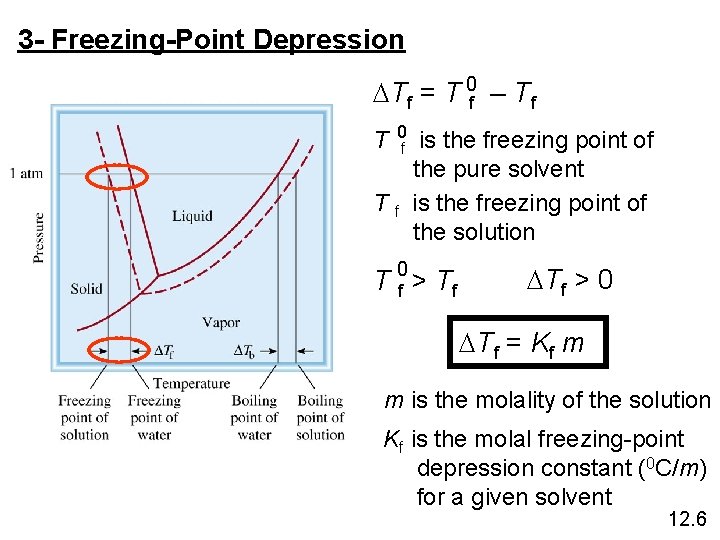
3 - Freezing-Point Depression DTf = T 0 f – Tf T 0 f is the freezing point of the pure solvent T f is the freezing point of the solution T 0 f > Tf DTf > 0 DTf = Kf m m is the molality of the solution Kf is the molal freezing-point depression constant (0 C/m) for a given solvent 12. 6

12. 6

What is the freezing point of a solution containing 478 g of ethylene glycol (antifreeze) in 3202 g of water? The molar mass of ethylene glycol is 62. 01 g. DTf = Kf m m = Kf water = 1. 86 0 C/m moles of solute mass of solvent (kg) 478 g x = 1 mol 62. 01 g 3. 202 kg solvent = 2. 41 m DTf = Kf m = 1. 86 0 C/m x 2. 41 m = 4. 48 0 C DTf = T 0 f – Tf Tf = T 0 f – DTf = 0. 00 0 C – 4. 48 0 C = -4. 48 0 C 12. 6

Worked Example 12. 8

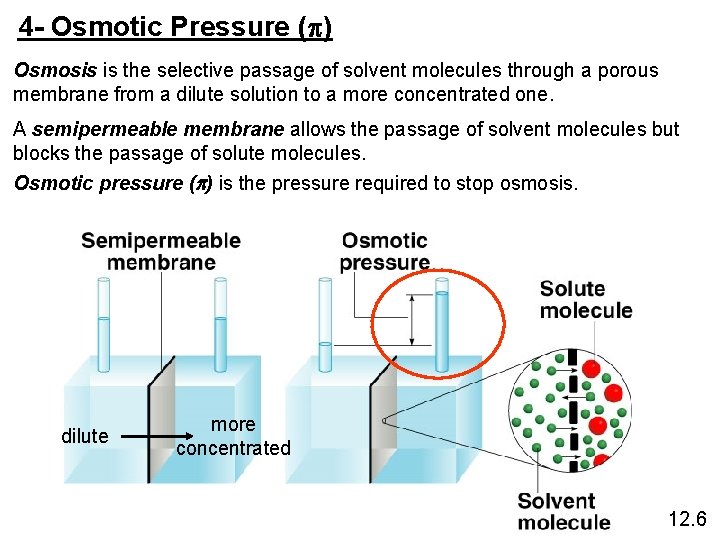
4 - Osmotic Pressure (p) Osmosis is the selective passage of solvent molecules through a porous membrane from a dilute solution to a more concentrated one. A semipermeable membrane allows the passage of solvent molecules but blocks the passage of solute molecules. Osmotic pressure (p) is the pressure required to stop osmosis. dilute more concentrated 12. 6

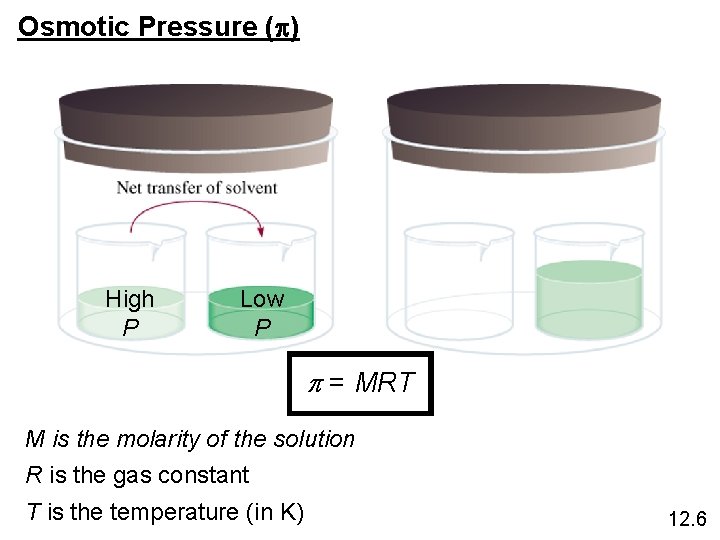
Osmotic Pressure (p) High P Low P p = MRT M is the molarity of the solution R is the gas constant T is the temperature (in K) 12. 6

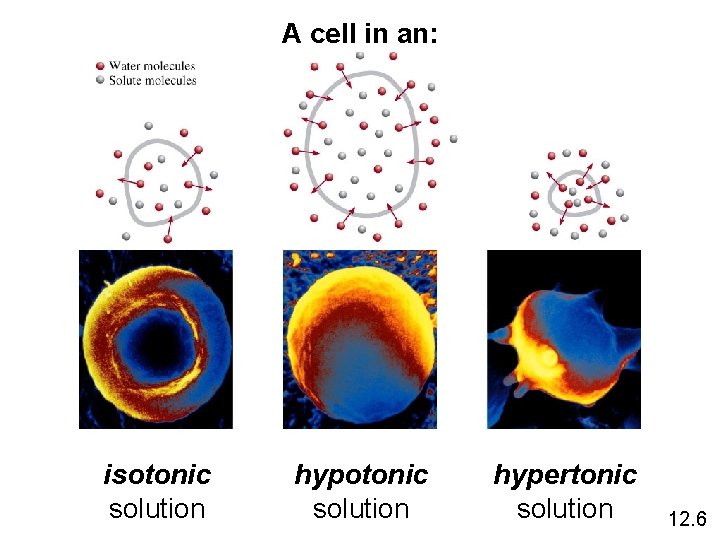
A cell in an: isotonic solution hypertonic solution 12. 6

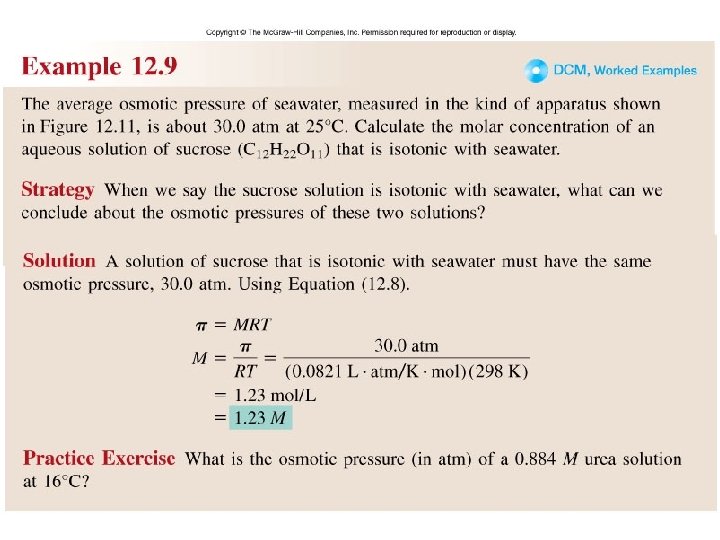
Worked Example 12. 9

Colligative Properties of Nonelectrolyte Solutions Colligative properties are properties that depend only on the number of solute particles in solution and not on the nature of the solute particles. Vapor-Pressure Lowering P 1 = X 1 P 10 Boiling-Point Elevation DTb = Kb m Freezing-Point Depression DTf = Kf m Osmotic Pressure (p) p = MRT 12. 6

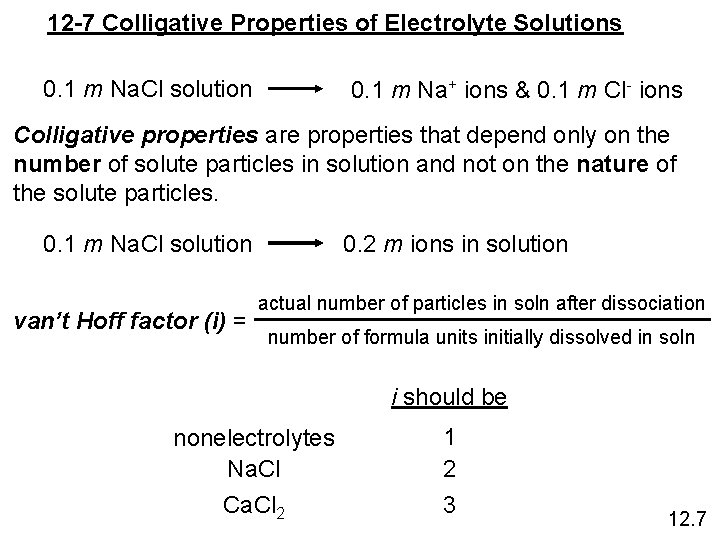
12 -7 Colligative Properties of Electrolyte Solutions 0. 1 m Na. Cl solution 0. 1 m Na+ ions & 0. 1 m Cl- ions Colligative properties are properties that depend only on the number of solute particles in solution and not on the nature of the solute particles. 0. 1 m Na. Cl solution van’t Hoff factor (i) = 0. 2 m ions in solution actual number of particles in soln after dissociation number of formula units initially dissolved in soln i should be nonelectrolytes Na. Cl Ca. Cl 2 1 2 3 12. 7

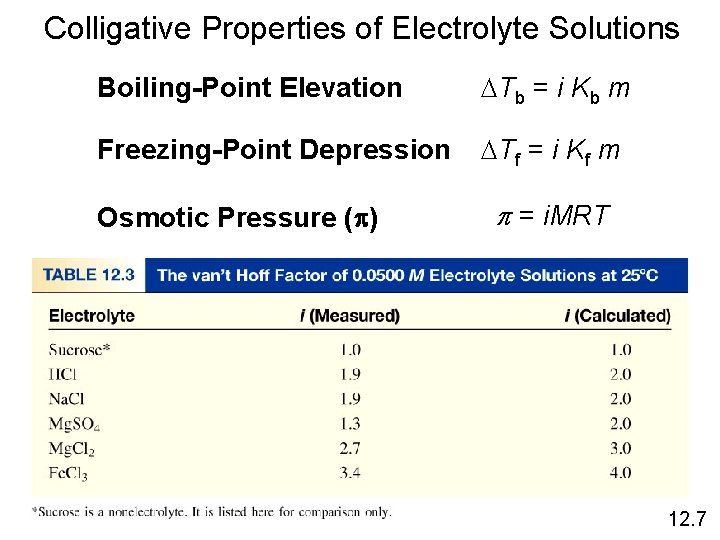
Colligative Properties of Electrolyte Solutions Boiling-Point Elevation DTb = i Kb m Freezing-Point Depression DTf = i Kf m Osmotic Pressure (p) p = i. MRT 12. 7

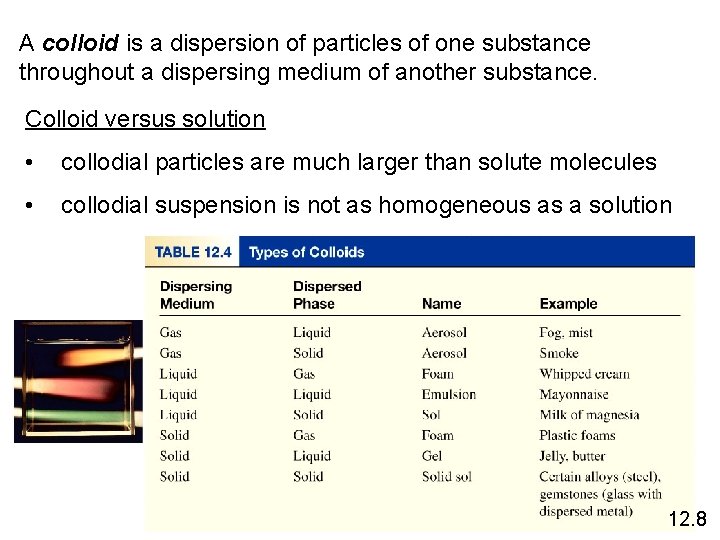
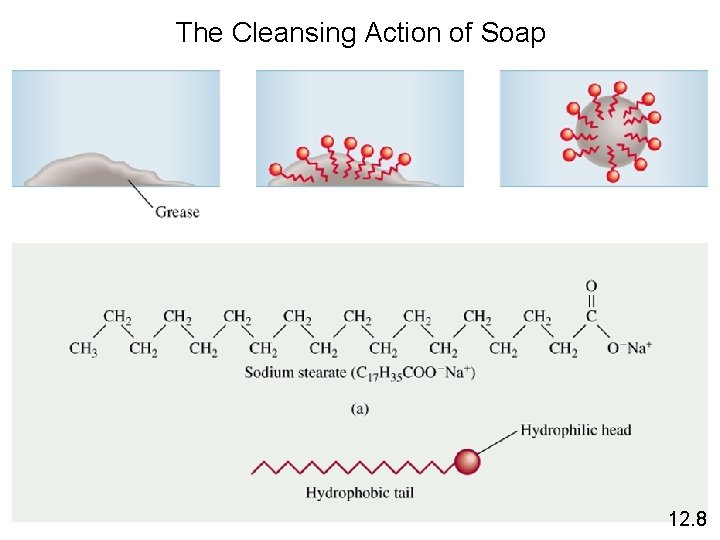
A colloid is a dispersion of particles of one substance throughout a dispersing medium of another substance. Colloid versus solution • collodial particles are much larger than solute molecules • collodial suspension is not as homogeneous as a solution 12. 8

The Cleansing Action of Soap 12. 8

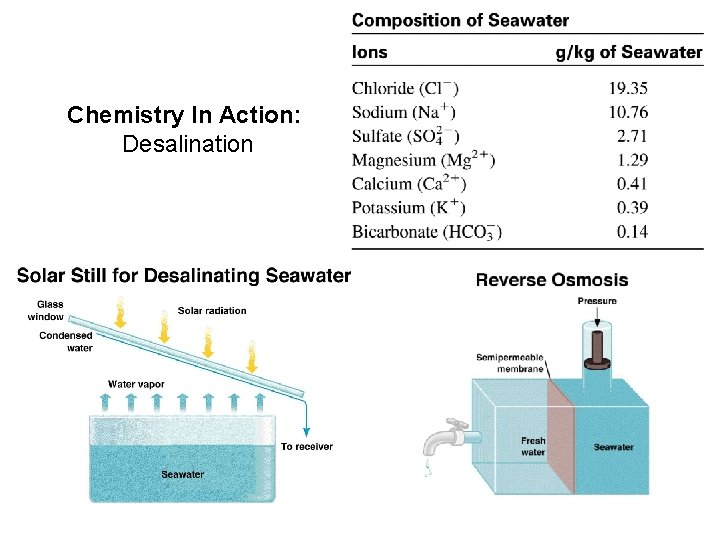
Chemistry In Action: Desalination

 Physical properties of solutions
Physical properties of solutions Physical properties of solutions
Physical properties of solutions Chemical and physical properties
Chemical and physical properties Chapter 13 properties of solutions
Chapter 13 properties of solutions Reocentral
Reocentral Solutions grade 7
Solutions grade 7 Freezing point chapter 13
Freezing point chapter 13 Which solution
Which solution General properties of aqueous solutions
General properties of aqueous solutions Visitor management solutions for properties
Visitor management solutions for properties Extensive vs intensive properties
Extensive vs intensive properties Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worms-breton
Tư thế worms-breton Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng chữ f
Môn thể thao bắt đầu bằng chữ f Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Ví dụ giọng cùng tên
Ví dụ giọng cùng tên Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế




























































