Physical Properties of Solutions Chapter 12 1 Copyright















- Slides: 26

Physical Properties of Solutions Chapter 12 1 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

A solution is a homogenous mixture of 2 or more substances The solute is(are) the substance(s) present in the smaller amount(s) The solvent is the substance present in the larger amount 2

A saturated solution contains the maximum amount of a solute that will dissolve in a given solvent at a specific temperature. An unsaturated solution contains less solute than the solvent has the capacity to dissolve at a specific temperature. A supersaturated solution contains more solute than is present in a saturated solution at a specific temperature. Sodium acetate crystals rapidly form when a seed crystal is added to a supersaturated solution of sodium acetate. 3

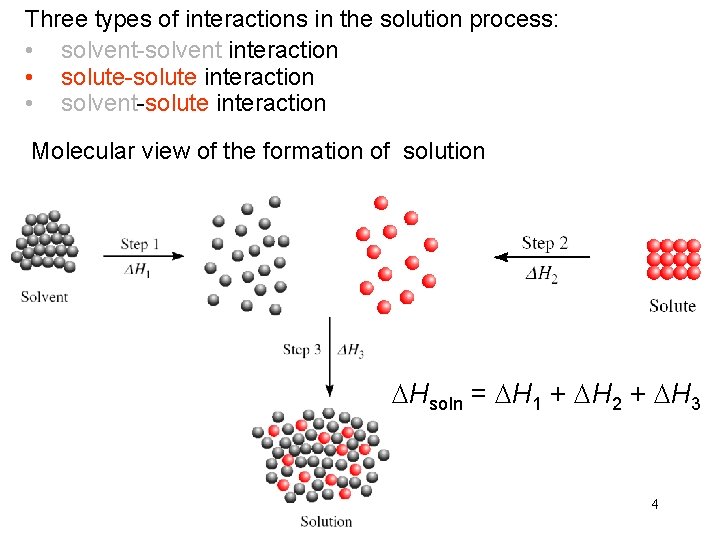
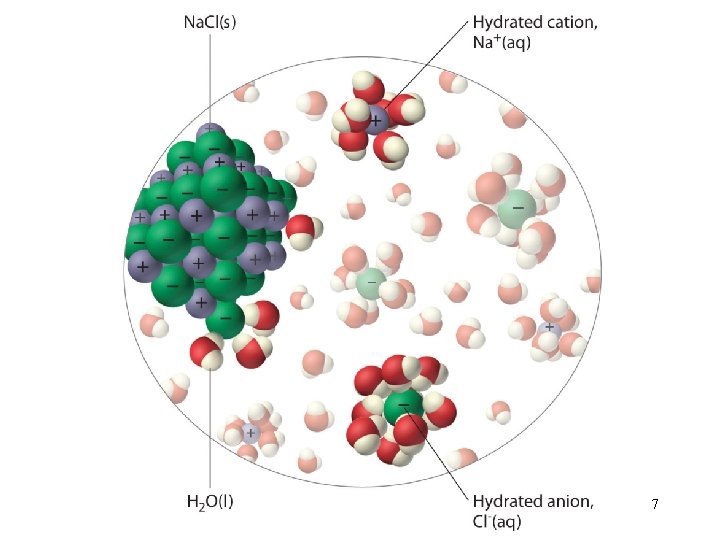
Three types of interactions in the solution process: • solvent-solvent interaction • solute-solute interaction • solvent-solute interaction Molecular view of the formation of solution DHsoln = DH 1 + DH 2 + DH 3 4

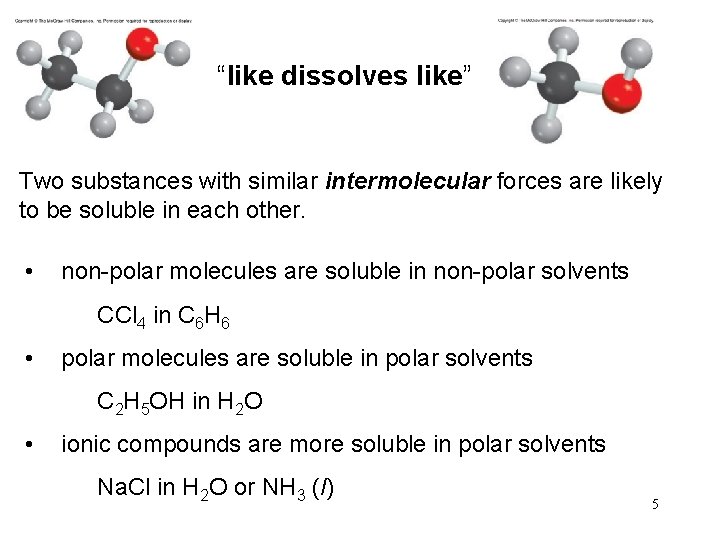
“like dissolves like” Two substances with similar intermolecular forces are likely to be soluble in each other. • non-polar molecules are soluble in non-polar solvents CCl 4 in C 6 H 6 • polar molecules are soluble in polar solvents C 2 H 5 OH in H 2 O • ionic compounds are more soluble in polar solvents Na. Cl in H 2 O or NH 3 (l) 5

6

7

Review of Concepts Which of the following would you expect to be more soluble in benzene than in water: C 4 H 10, HBr, KNO 3, P 4 8

Example 12. 1 Pg 523 Predict the relative solubilities in the following cases: (a) Bromine (Br 2) in benzene (C 6 H 6, = 0 D) and in water ( = 1. 87 D) (b) KCl in carbon tetrachloride (CCl 4, = 0 D) and in liquid ammonia (NH 3, = 1. 46 D) (c) formaldehyde (CH 2 O) in carbon disulfide (CS 2, = 0 D) and in water

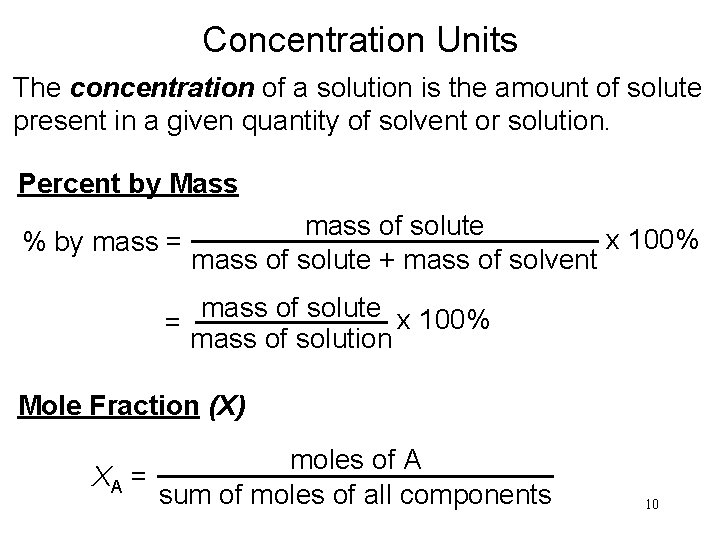
Concentration Units The concentration of a solution is the amount of solute present in a given quantity of solvent or solution. Percent by Mass mass of solute x 100% % by mass = mass of solute + mass of solvent mass of solute x 100% = mass of solution Mole Fraction (X) moles of A XA = sum of moles of all components 10

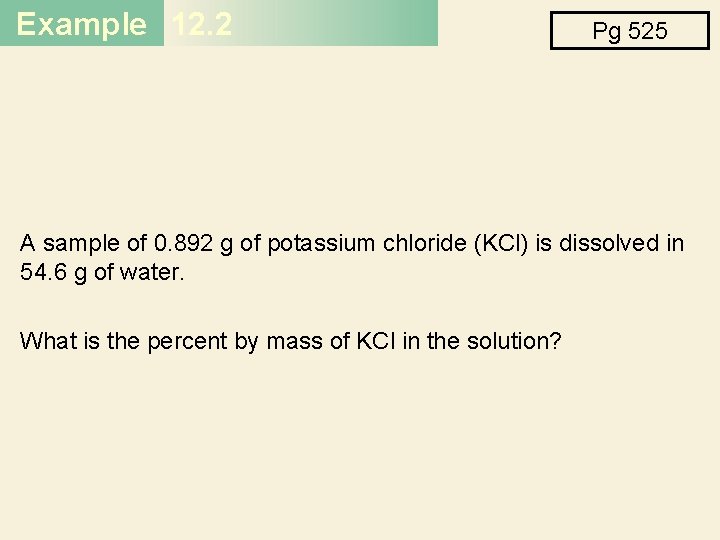
Example 12. 2 Pg 525 A sample of 0. 892 g of potassium chloride (KCl) is dissolved in 54. 6 g of water. What is the percent by mass of KCl in the solution?

Concentration Units Molarity (M) M = moles of solute liters of solution 12

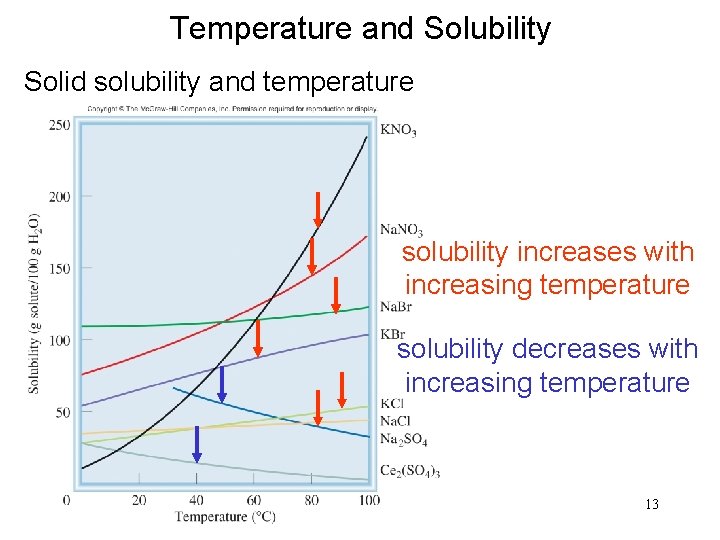
Temperature and Solubility Solid solubility and temperature solubility increases with increasing temperature solubility decreases with increasing temperature 13

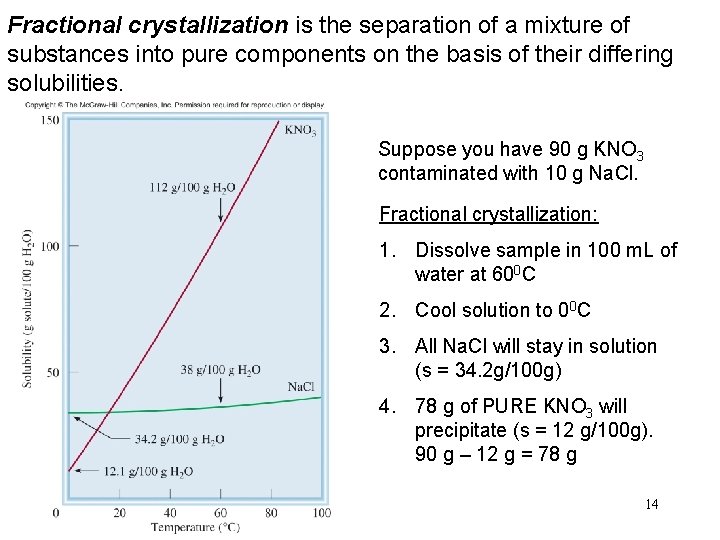
Fractional crystallization is the separation of a mixture of substances into pure components on the basis of their differing solubilities. Suppose you have 90 g KNO 3 contaminated with 10 g Na. Cl. Fractional crystallization: 1. Dissolve sample in 100 m. L of water at 600 C 2. Cool solution to 00 C 3. All Na. Cl will stay in solution (s = 34. 2 g/100 g) 4. 78 g of PURE KNO 3 will precipitate (s = 12 g/100 g). 90 g – 12 g = 78 g 14

Temperature and Solubility O 2 gas solubility and temperature solubility usually decreases with increasing temperature 15

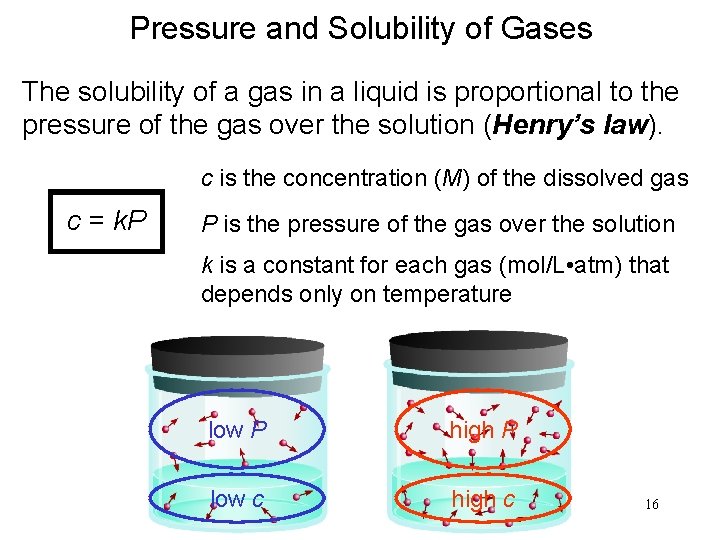
Pressure and Solubility of Gases The solubility of a gas in a liquid is proportional to the pressure of the gas over the solution (Henry’s law). c is the concentration (M) of the dissolved gas c = k. P P is the pressure of the gas over the solution k is a constant for each gas (mol/L • atm) that depends only on temperature low P high P low c high c 16

Colligative Properties Colligative properties are properties that depend only on the number of solute particles in solution and not on the nature of the solute particles. Vapor-Pressure Lowering Adding a nonvolatile solute reduces the relative amount of solvent, which results in fewer molecules becoming vapor % 0 10 nt e solv % 0 <10 nt e v l o s 17

Fractional Distillation Apparatus 18

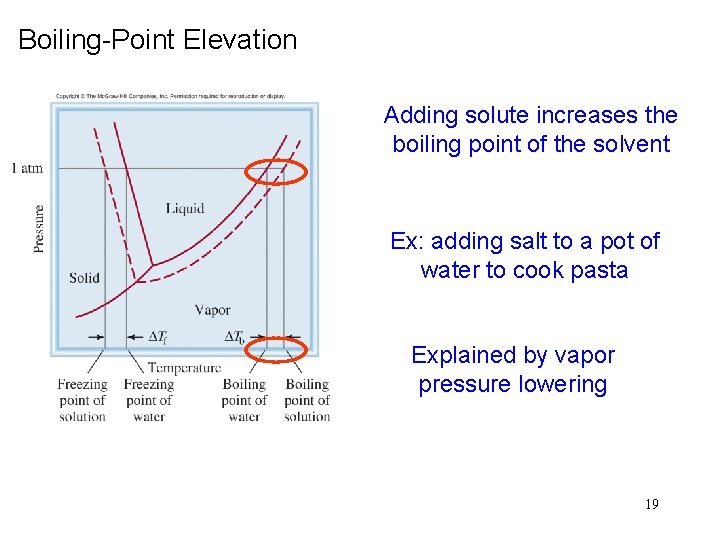
Boiling-Point Elevation Adding solute increases the boiling point of the solvent Ex: adding salt to a pot of water to cook pasta Explained by vapor pressure lowering 19

Freezing-Point Depression Adding solute lowers the freezing point of the solvent Ex: salt or sand on roads to prevent ice 20

Effect of Concentration Solute Added to Water Resulting Freezing Point Just water 0°C 1 mol sugar − 1. 86°C 1 mol Na. Cl − 3. 72°C 1 mol Ca. Cl 2 − 5. 78°C 21

22

A colloid is a dispersion of particles of one substance throughout a dispersing medium of another substance. Colloid versus solution • colloidal particles are much larger than solute molecules • colloidal suspension is not as homogeneous as a solution • colloids exhibit the Tyndall effect 23

24

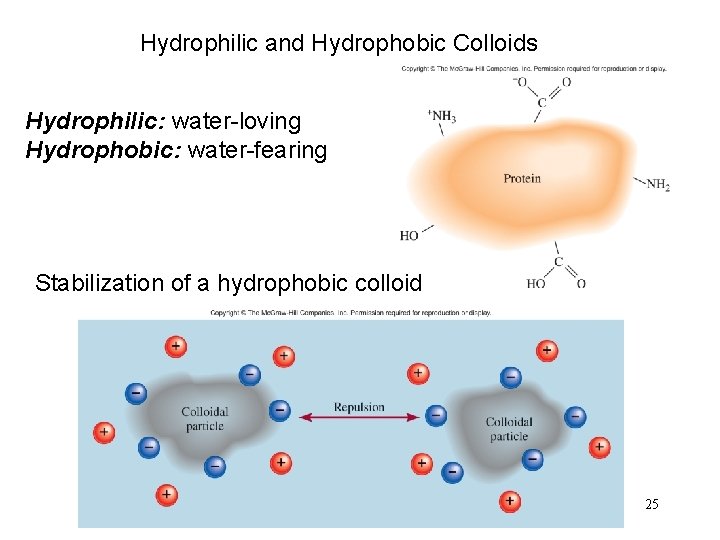
Hydrophilic and Hydrophobic Colloids Hydrophilic: water-loving Hydrophobic: water-fearing Stabilization of a hydrophobic colloid 25

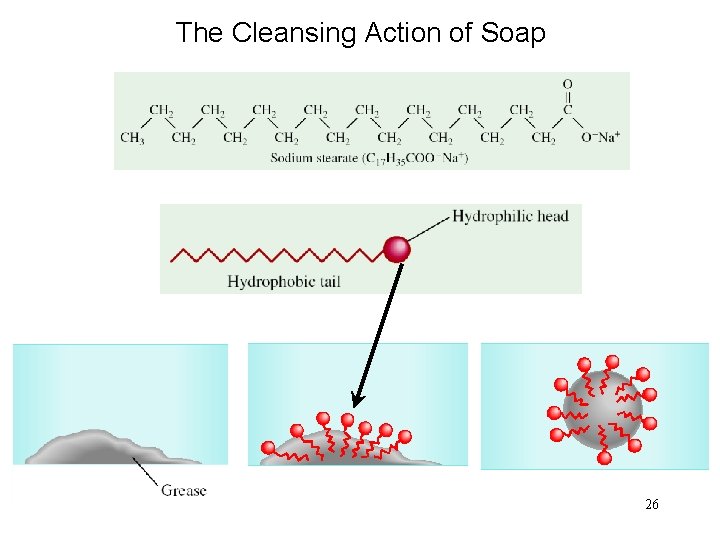
The Cleansing Action of Soap 26
 Physical properties of solutions
Physical properties of solutions Physical properties of solutions
Physical properties of solutions Chemical property of matter
Chemical property of matter Chapter 13 properties of solutions
Chapter 13 properties of solutions Reo management solutions llc
Reo management solutions llc What is a solute in science grade 7
What is a solute in science grade 7 Ions in aqueous solutions and colligative properties
Ions in aqueous solutions and colligative properties 16.3 colligative properties of solutions
16.3 colligative properties of solutions General properties of aqueous solutions
General properties of aqueous solutions Visitor management solutions for properties
Visitor management solutions for properties Intensive and extensive properties
Intensive and extensive properties Physical asset management solutions
Physical asset management solutions Amino acid optical isomers
Amino acid optical isomers Addition polymerisation animation
Addition polymerisation animation Physical and chemical properties of sulphuric acid
Physical and chemical properties of sulphuric acid 2 hydrogen 1 oxygen
2 hydrogen 1 oxygen A scientist performs an experiment, and an actor performs a
A scientist performs an experiment, and an actor performs a Physical property of starch
Physical property of starch Physical properties of ocean water
Physical properties of ocean water Chemical properties of dental materials
Chemical properties of dental materials Lesson outline physical properties lesson 2
Lesson outline physical properties lesson 2 Physical properties of cardboard
Physical properties of cardboard Chemical properties
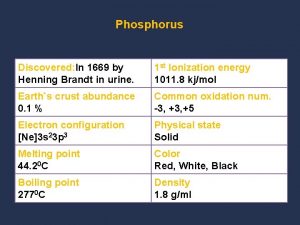
Chemical properties Chemical properties of phosphorus
Chemical properties of phosphorus Physical properties of paint
Physical properties of paint The physical properties of metals include luster and
The physical properties of metals include luster and Defintion of matter
Defintion of matter