PHYSICAL PROPERTIES OF DENTAL MATERIALS OUTLINE n What































![Thermal Conductivity Of Dental Materials Material Thermal Conductivity (cat/sec/cm 2[o. C/cm]) Human enamel 0. Thermal Conductivity Of Dental Materials Material Thermal Conductivity (cat/sec/cm 2[o. C/cm]) Human enamel 0.](https://slidetodoc.com/presentation_image_h2/4f26b26ec54fad8e9778d9dc2eecd82c/image-37.jpg)



















- Slides: 60

PHYSICAL PROPERTIES OF DENTAL MATERIALS

OUTLINE n What are physical properties? n ABRASION AND ABRASION RESISTANCE n VISCOSITY n STRUCTURAL AND STRESS RELAXATION n CREEP AND FLOW n COLOUR AND COLOUR PERCEPTION n THERMO PHYSICAL PROPERTIES n INTRODUCTION TO TARNISH AND CORROSION

CAUSES OF TARNISH AND CORROSION n CLASSIFICATION OF CORROSION n ELECTROCHEMICAL CORROSION n METHODS TO PREVENT CORROSION n

What are physical properties? Physical properties are based on the laws of mechanics acoustics optics thermodynamic magnetism Electricity Radiation Atomic structure Nuclear phenomena

Hue, value and chroma are physical properties that are based on the laws of optics, which is the science that deals with phenomena of light, vision and sight.

Thermal conductivity and coefficient of thermal expansion are physical properties that are based on the laws of thermodynamics.

viscosity, which is the resistance of a fluid to flows is related to the fields of material science and mechanics.

Abrasion and Abrasion resistance n n n Hardness has often been used as an index of the ability of a material to resist abrasion or wear. Abrasion is a complex mechanisim in the oral environment that involves an interaction among numerous factors. Hardness may be useful for comparing materials within a given classification, such as one brand of cast metal with other brand of the same type casting alloy.

Indentation Method : n (a) Brinell hardness test n (b) Knoop hardness test n (c) Vickers hardness test n (d) Rockwell hardness test n (e) Shore A hardness test Each of these tests qiffers slightly from the other. Brinell and Rockwell tests are classified as macrohardness tests. Knoop and Vickers tests as microhardness tests.

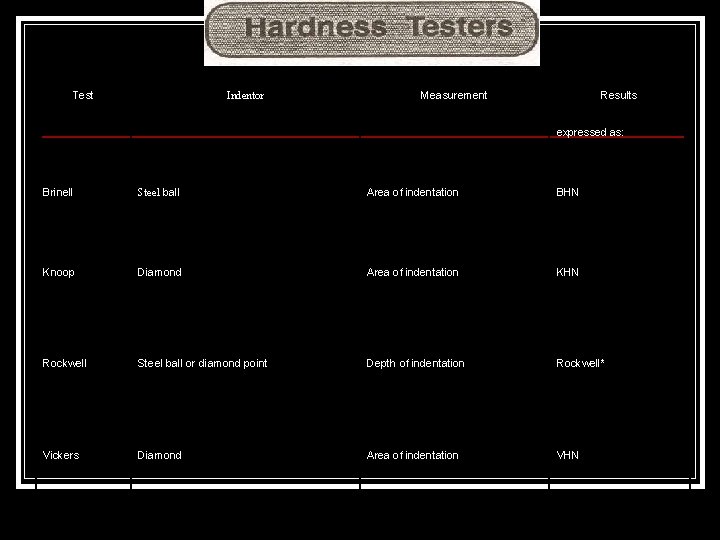
Test Indentor Measurement Results expressed as: Brinell Steel ball Area of indentation BHN Knoop Diamond Area of indentation KHN Rockwell Steel ball or diamond point Depth of indentation Rockwell* Vickers Diamond Area of indentation VHN

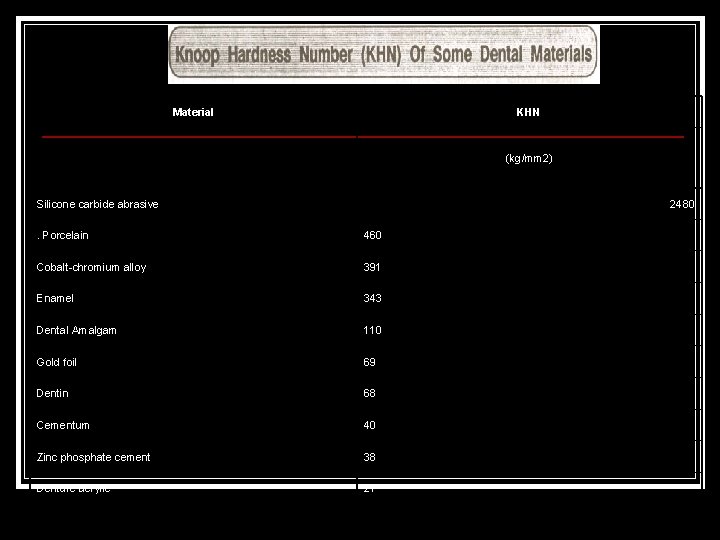
Material KHN (kg/mm 2) Silicone carbide abrasive 2480 . Porcelain 460 Cobalt chromium alloy 391 Enamel 343 Dental Amalgam 110 Gold foil 69 Dentin 68 Cementum 40 Zinc phosphate cement 38 Denture acrylic 21

Cobalt chromium alloy 391 Enamel 343 Dental Amalgam 110 Gold foil 69 Dentin 68 Cementum 40 Zinc phosphate cement 38 Denture acrylic 21

Viscosity n n n Viscosity is the resistance of a liquid to flow. The success or failure of a given material may be as dependent on its properties in the liquid state as it on its properties as a solid. For example, materials like cements and impression materials undergo liquid to solid transformation in the mouth.

Gypsum products used in the fabrication of models and dies are transformed from slurries into a solid structures. n Amorphous materials such as waxes and resins appear solids but actually are super cooled liquids that can flow plastically (irreversibly) under sustained loading or deformed elastically (reversibly) under small stresses. n

A liquid occupies space between 2 metals plates; the lower plate is fixed and upper plate is moved right at a velocity (V). A force (F) is required to overcome the frictional resistance to the fluid flow. If the plates have an area (A) in contact with the liquid, a Shear stress ( ) ז can be defined as = ז F/A. The shear strain rate or the rate of change of deformation, is έ=V/d, where d is the shear distance of top plate relative to the fixed lower plate.

As the shear force (F) increases, V increases and a curve can be obtained for a force Vs Velocity


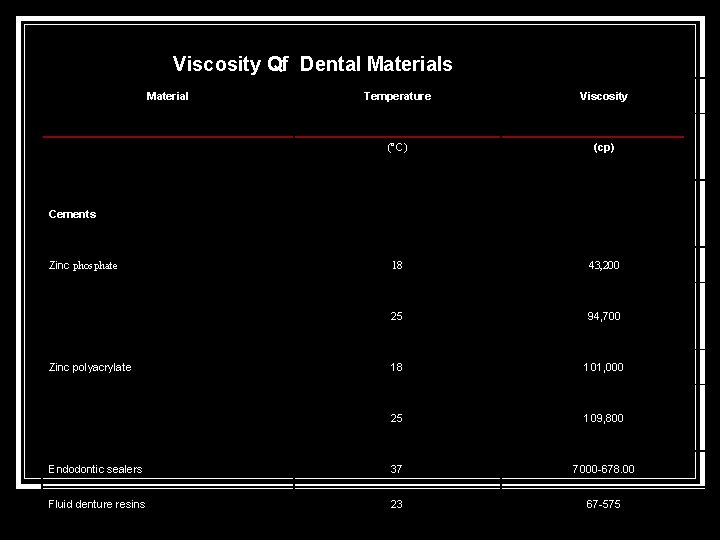
Viscosity Qf Dental Materials Material Temperature Viscosity (°C) (cp) 18 43, 200 25 94, 700 18 101, 000 25 109, 800 Endodontic sealers 37 7000 678. 00 Fluid denture resins 23 67 575 Cements Zinc phosphate Zinc polyacrylate

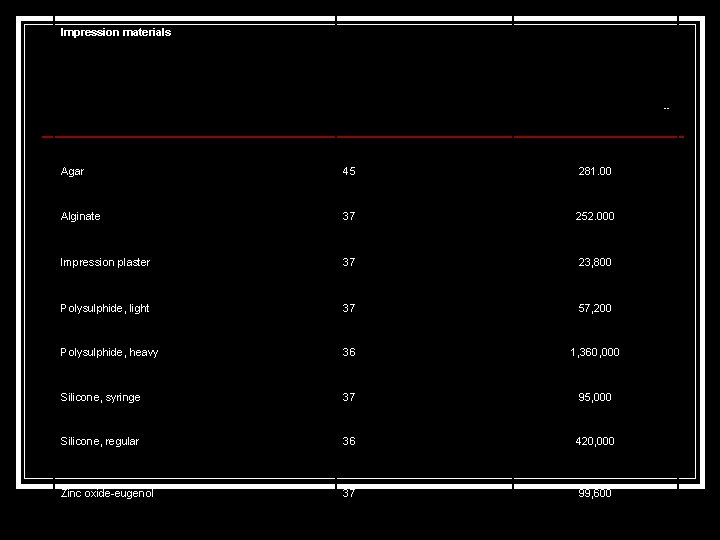
Impression materials -- Agar 45 281. 00 Alginate 37 252. 000 Impression plaster 37 23, 800 Polysulphide, light 37 57, 200 Polysulphide, heavy 36 1, 360, 000 Silicone, syringe 37 95, 000 Silicone, regular 36 420, 000 Zinc oxide eugenol 37 99, 600

Viscoelasticity Many materials are intermediate between that of a viscous liquid an elastic solid. This phenomena is termed viscoelasticity. The rate of loading is important for many dental materials, by increasing the loading (strain) rate produces a different stress strain curve. With higher rates giving higher values for the elastic modulus, proportional limit and ultimate strength. Materials that have mechanical properties independent of loading rate are termed elastic. Materials that have mechanical properties dependent on loading rate are viscoelastic.

Viscoelastic. . properties are of major importance for a group of dental materials, for example: Elastomericimpression materials. To avoid excess perma'nent deformation of these materials, they shouldnot be loaded for any longer than necessary, must be removed from the mouth with a short sharp pull. The more rapid the material is loaded and unloaded the more elastically the material will respond_

Structural and Stress relaxation After a substance has been permanently deformed, there are trapped internal stresses. For example, in a crystalline substance the atoms in the space lattice are displaced, and the system is not in equilibrium. similarly, in amorphous structure some molecules are too close together and others too far apart after the substance has been permanently deformed.

Creep and Flow Creep: is defined as the time dependent plastic strain of a material under a static load or constant stress Flow: It is somewhat similar to creep. The term flow is used instead of creep to describe rheology of amorphous materials, e. g. waxes. Waxes deform under a small static load, even that associated with its own mass. Although creep and flow may be measured under any type of stress, compression is usually used for testing of dental materials.

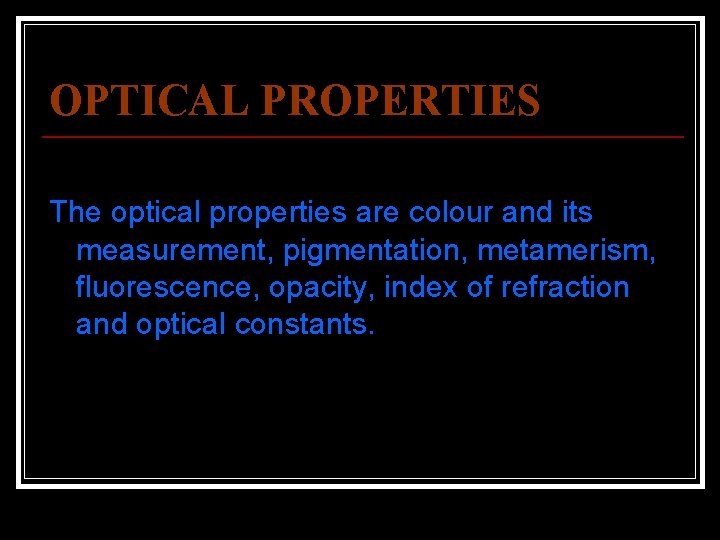
OPTICAL PROPERTIES The optical properties are colour and its measurement, pigmentation, metamerism, fluorescence, opacity, index of refraction and optical constants.

Colour and Colour perception Light is electromagnetic radiation that can be detected by the human eye. The eye is sensitive to wavelength from approx 400(violet) to 700 nanometer (dark red). for an object to be visible it must reflect or transmit light incident on it from an external source. The latter is the case for the objects that are of dental interest.

Light from an object that is incident on an eye is focused on the retina and is converted into a nerve impulse that are transmitted to the brain. Cone shaped cells in the retina are responsible for colour vision. Eye is most sensitive to the light in the green yellow region and least sensitive at either extreme i. e red or blue. Measurement of colour One of the most commonly used method to define and measure colourquantitatively is the Munsell System. It is a co ordinate system which can be viewed as a cylinder. The lines are arranged sequentially around the perimeter of the cylinder, while the chroma increases along a radius from the axis. The value coordinate varies along the length of the cylinder from black at the bottom to neutral grey at the center to white at the top.

Three dimensions of colour Hue n Value n Chroma n

Hue describes the dominant colour of an object. Eg: red, green or blue. Value is the lightness or darkness of colour which can be measured independent of Hue. Eg: the yellow of the lemon is lighter than the red of a cherry. Chroma represents degree of saturation of of a particular hue.




Metamerism The quality and intensity of light must be controlled in matching colours in dental restorations. Because light from incandescent lamps, fluorescent lamps and the sun differs. Objects that appear to be colour matched under one type of light may appear very different under another light source. For this reason, it is important that colour matching should be carried out under a variety of the light sources, with bright daylight being by far the best. The fact that objects can change colour under the influence of different light sources is known as metamerism.

n n n Fluorescence One of the important feature of light is that some objects are able to absorb light of a wavelength near ultraviolet range (300 400 nm), and then release it as light of longer wavelength (400 450 nm). This is the property of fluorescence, it occurs in natural tooth enamel. This is the reason why natural teeth look so white under a fluorescent light and why some crowns, bridges or fillings are more noticeable in such light, as they do not fluoresce, and look dark next to the fluorescing natural tooth. Blue or ultraviolet light produces fluorescent light that is in the visible range.

Opacity, Translucency And Transparency Opacity is a property of materials that prevents the passage of light Le. , opaque material is one that does not transmit light. but instead absorbs light or reflects or scatters from the surface. The colour of the object will depend on which wavelengths of light are reflected and which are absorbed. A translucent material allows some light to pass through it, absorbs some of the light and scatters and reflects the rest from its surface. An object viewed through such a material would have a distorted appearance. Some translucent materials used in dentistry are porcelain. composite resins and dental plastics. A transparent material allows passage of light in such a way that little distortion takes place, meaning that an object can be seen quite clearly through it. Selective absorption of certain wavelengths may take place and it forms the basis for optical filters. For example, red glass is red because it allows light with the wavelength of red light to pass through it but abosrbs all other wavelengths. And, it would appear opaque if the light source does not contain light with the wavelength of red light, since all the other wavelengths are absorbed.

THERMAL PROPERTIES Materials in the mouth are subjected to variations in temperature because of the intake of hot foods and drinks. The response of a material to a source of heat depends upon the ease with which heat is transferred through the material. In dentistry measurement of heat is done during cavity cutting, with a thermometer or a thermocouple.

Thermal Conductivity n n Thermal conductivity is defined as the quantity of heat passing per second between the opposite faces of a unit cube when the difference in temperature is unity. A material through which heat is transferred easily is known as good thermal conductor (K). Materials with good thermal conductivity values are good conductors of heat and cold. A material which resists. the conduction of heat is a thermal insulator Materials have different rates of conducting heat. The units are cal/sec/cm 2 e. C/cm) Restorative materials should be a bad conductor of heat, so that hot and cold sensations do not pass through them and do not produce pulpal damage. Denture base materials should be good conductor of heat, otherwise sensations of cold cannot be felt by the patient. Metals have higher values than plastics and ceramics. Human enamel and dentin are poor conductor. When a portion of tooth is replaced by a metal restoration such as amalgam or gold alloy, the tooth may be temporarily sensitive to temperature changes in the mouth. In deep cavity preparations thermal insulating bases are used and under metal restorations, the reason being although dentin is a poor thermal conductor, a thin layer of it does not provide enough thermal insulation for the pulp unless a cement base is used. . Composites have thermal conductivity similar to that of tooth structure so they do not present a problem with this property.
![Thermal Conductivity Of Dental Materials Material Thermal Conductivity catseccm 2o Ccm Human enamel 0 Thermal Conductivity Of Dental Materials Material Thermal Conductivity (cat/sec/cm 2[o. C/cm]) Human enamel 0.](https://slidetodoc.com/presentation_image_h2/4f26b26ec54fad8e9778d9dc2eecd82c/image-37.jpg)
Thermal Conductivity Of Dental Materials Material Thermal Conductivity (cat/sec/cm 2[o. C/cm]) Human enamel 0. 0022 Human dentin 0. 0015 Dental amalgam 0. 055 Composite plastics 0. 0025 Gold alloys 0. 710 Unfilled acrylic plastics 0. 0005 Porcelain 0. 025 Zinc Phosphate cement 0. 0028 Zinc oxide eugenol cement 0. 0011

n Specific Heat: For some materials, the initial 'cold feeling' c(; !n rapidly disappear as the material heats up due to the transfer of heat energy from the heat source to the material. How rapidly the temperature increases depends upon the specific heat, cp, is the quantity of heat needed to rise the temperature of one gram of the substance by one degree centigrade We). The units are cal/gmr. C. For example, because of the difference in the specific heat of water and alcohol, 100 gms of water require more heat than 100 gms of alcohol to rise the temperature to one degree centigrade. The specific heat of gold and the metals used in gold alloys is low. so prolonged heating during melting and casting procedure is unnecessary. The specific h. eat of both enamel and dentin is higher that the. restorative materials like amalgam and gold alloys.

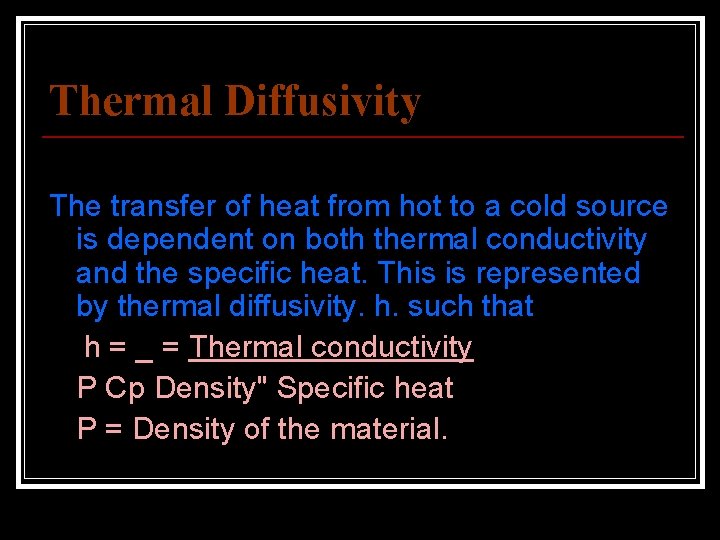
Thermal Diffusivity The transfer of heat from hot to a cold source is dependent on both thermal conductivity and the specific heat. This is represented by thermal diffusivity. h. such that h = _ = Thermal conductivity P Cp Density" Specific heat P = Density of the material.

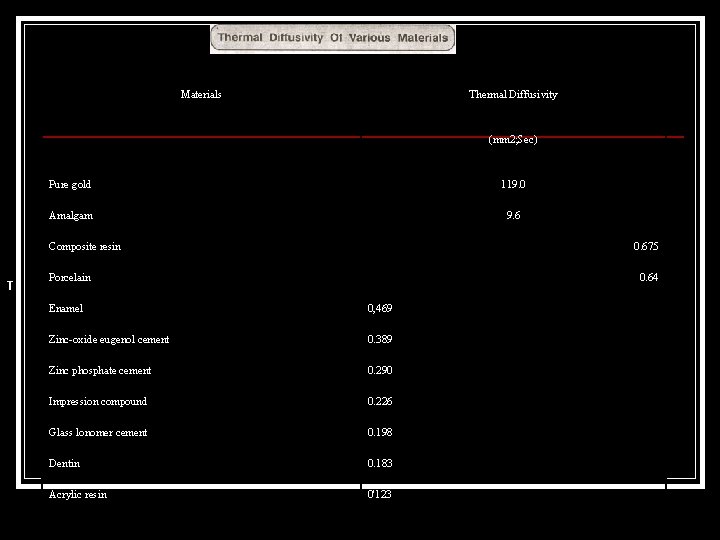
Thermal conductivity (K) regulates the rate at which the heat enters andpasses through the material. Specific heat (Cp) determines the rate at which the temperature will riseas heat enters the material. The thermal diffusivity gives a clear indicatioh of the rate of rise of temperature at one point due to a heat source at another point. The unit of thermal diffusivity are mm 2; Sec. The low specific heat of a gold inlay or crown or a dental amalgam, combined with the high thermal conductivity creates a thermal shock more than the normal tooth structure does.

Materials Thermal Diffusivity (mm 2; Sec) Pure gold 119. 0 Amalgam 9. 6 Composite resin T 0. 675 Porcelain 0. 64 Enamel 0, 469 Zinc-oxide eugenol cement 0. 389 Zinc phosphate cement 0. 290 Impression compound 0. 226 Glass lonomer cement 0. 198 Dentin 0. 183 Acrylic resin 0'123

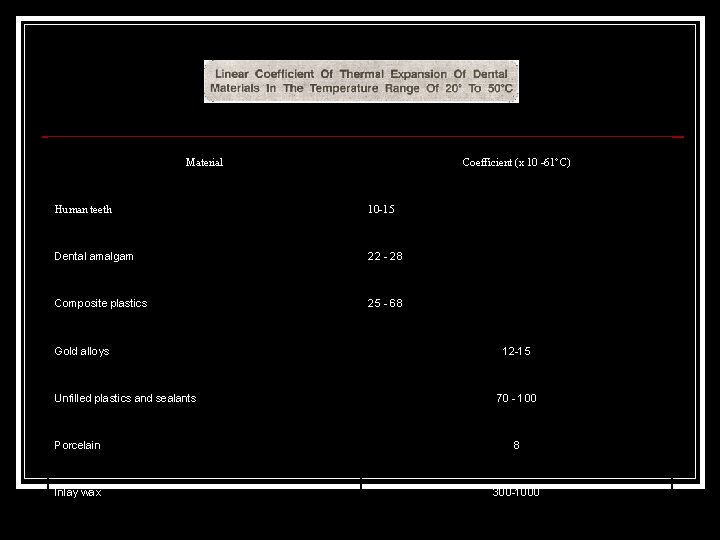
Thermal Expansion Restorative dental materials in the mouth are subjected to temperature changes, as a consequence the material expands. Thermal expansion of the restorative materials usually does not match that of the tooth structure, a differential expansion occurs. The most common way of measuring this expansion is by taking a length of material, heating it to a certain temperature and then measuring the resultant change in length. Change in per unit length for a 1°C change in temperature, is called the linear coefficient of thermal expansion, a. This is expressed as parts per millionr. C.

Material Coefficient (x 10 -61°C) Human teeth 10 -15 Dental amalgam 22 28 Composite plastics 25 68 Gold alloys Unfilled plastics and sealants 12 15 70 100 Porcelain 8 Inlay wax 300 1000

n Tarnish is the surfa. ; e discolouration on a metal or slight loss of surface lustre or finish. Tarnish in the oral cavity is caused due to : The formation of hard and soft deposits on the surface of the restoration like calculus (hard deposit), changes the colour from light yellow to brown or still darker, plaque (soft deposit), is mainly formed by micro organisms, and mucin. Some pigment producing bacteria causes discolouration. Some drugs containing chemicals like iron, mercury and the absorbed food , debris. Oxides, sulphides or chlorides forms a thin film leading to discolouration. The discolouration resulting from tarnish can be easily removed from the surface by polishing the metal.

Corrosion is not a surface discolouration but is the dissolution of metals in the mouth. Corrosion can be defined as a chemical reaction between a metal and its environment to form metal compound. Corrosion, therefore, is potentially a much more serious problem than tarnish. Example of corrosion is rusting of iron. Iron combines with both, the oxygen in air and water to form a hydrated oxide of iron (Fe 203' H 2 O). Usually the corrosion product of a metal is very similar to the compound from which it is formed. Iron is extracted from naturally occuring iron oxide, and rust is simply hydrated iron oxide. Corrosion is obviously undersirable. as it can spoil the esthetics of an alloy and can severely weaken the material. Corrosion rate increases with time. The process of corrosion may be accelerated in the following conditions: _

CLASSIFICATION OF CORROSION There are generally two types of corrosion n (a) Non Aqueous Corrosion Or Chemical Or Dry Corrosion Such corrosion occurs in the absence of water or other fluid electrolytes hence it is called as dry corrosion. 'n chemical corrosion metals can react to form compounds such as oxides and sulphides. . Other than gold and a few other noble metals, all metals will form oxide layer when the surface comes into contact with the oxygen in air. Fe Fe 2+ + 2 e 4 e + ° 2 202. Fe I I Fe or Fe 203

n (b) Aqueous Corrosion or Electrolytic or Wet Corrosion Such corrosion occurs in the oral environment. The presence of moisture, temperature fluctuations, and the changing p. H caused by diet and decomposition of foodstuffs, contributes to this type of corrosion. This type of corrosion requires presence of water or other fluid electrolytes so it is termed as wet c. Orrosion. This occurs by electrochemical reactions. Pathway for the transport of electrons is required.

TYPES OF ELECTROCHEMICAL CORROSION n (1 ) Galvanic corrosion or Dissimilar metal corrosion n (2) Stress corrosion n (3) Concentration cell corrosion or Crevice corrosion

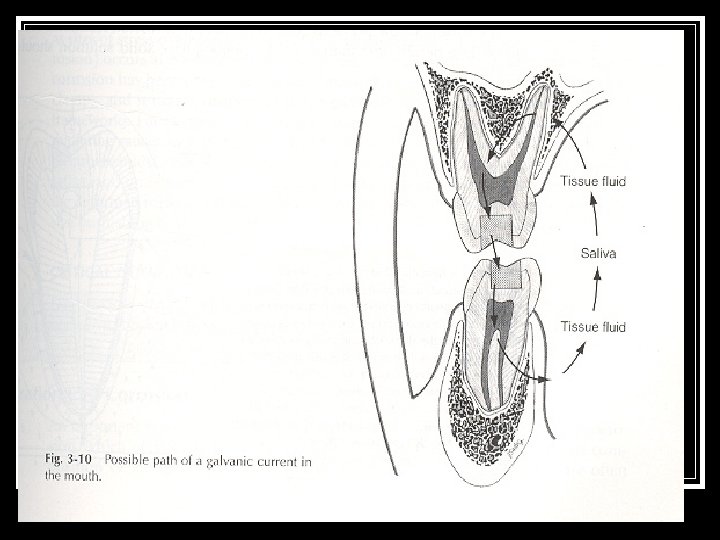
n (1) Galvanic' Corrosion or Dissimilar Metal Corrosion n Galvanism is the generation of electrical current that the patient can feel, which results when two dissimilar metals are in direct physical contact. Metals placed in an electrolyte have various tendencies to go into solution. Aluminum, alloys of which are sometimes used as temporary crowns has an electrode potential of + 1. 66 volts. Gold, has an electrode potential of - 1. 49 volts. These fillings in conjuction with saliva or oral fluids function as the electrolyte, acts similar to that of an electrical cell. When the two restorations comes in contact with each other, current flows because they are placed at different positions in electromotive series. If the flow of current occurs through the pulp, the patient experiences pain also known as galvanic pain and frequently complains of a metallic taste. Here, . the more anodic restoration may corrode. Gold alloy crown



n Different Conditions Where Galvanic Corrosion Occurs Corrosion can also occur when adjacent restorations are of dissimilar metals. As a result of the galvanic action, material goes into solution and roughness and pitting occurs. A single metallic restoration can show corrosion, between an external surface exposed to saliva and an interior surface exposed to dentinal fluid. Ions capable of conducting electricity can easily migrate.

n n n n n Single metallic restoration showing two possible current pathways between an external surface exposed to saliva and an interior surface exposed to dentinal fluid. Because the dentinal fluid contains a higher CI- concentration than does the saliva, it is assumed that the electrode potential of the interior surface exposed to dentinal fluid is more active; therefore, it is given a negative sign (-). The potential difference between the two surfaces is represented by E. . The same effect can be experienced when a tin foil, silver fork: spoon or any other metal comes in contact with the metal restoration. When the teeth are not in contact still a circuit develops between the two fillings, with surrounding tissues constituting the external circuit. * Galvanic currents are also developed between restorations of similar metals, as they differ in their surface composition or structure. Heterogenous Composition - Difference in the composition of materials It is a type of galvanic corrosion. (1) The eutectic and peritectic alloys are examples of this type of corrosion. The corrosion resistance of these alloys is less than solid solution. When an eutectic alloy is immersed in an electrolyte, the metallic grains with lower electrode potential are affected and the result is corrosion. (2) As compared to homogenized structure, cored structure is less resistant to corrosion. As in a cored structure, differences in the composition within alloy grains are found. Thus part of a grain can be the anode, and part cathode. Consequently, homogenization can help to improve the corrosion resistance of an alloy.

Stress Even a homogenized structure may corrode because of difference in structure between grain and their boundaries. The grain boundaries may act as anodes and the interior of the grain acts as a cathode. A soldered appliance or denture may corrode, since the composition of the solder differs from that of the alloy it joins. Impurities in any. alloy enhance corrosion.

n Corrosion (Differences in the stress) A metal which has been stressed by cold working, bending and burnishing, produces the localized stress in some part of the structure. If stressed and unstressed metals are in contact in an elecrolyte, the stressed area will become the anode of a galvanic cell and will corrode. So. excessive burnishing of metallic restoration should be avoided. In orthopedic surgery, stainless steel plates and screws are frequently embedded in tissues. Any differences in the extent of cold working between screws and plate must be avoided. Similarly, different portions of the same piece of steel may be stressed to different extends, thus results in corrosion.

Cencentration Cell Corrosion. or Crevice Corrosion (Difference in the Composition of Electrolyte) A homogeneous metal or alloy can undergo electrolytic corrosion where there is a difference in electrolyte concentration across the specimen examples: (1) Accumulation of food debris over a metallic restoration or in interproximal area. In such situation one type of electrolyte is formed under this debris and another electrolyte is saliva. So electrochemical corrosion occurs under the layer of food debris. (2) Where there is difference in the oxygen concentration in an electrolyte, n

METHODS TO PREVENT CORROSION n n n Noble metals such as gold, platinum, and palladium resist corrosion because their EMF is positive. ' Dental alloys should ideally contain at least 70 75% noble metals. Thus increasing the content of noble metals in dental alloys prevents corrosion. By coating the metal surface with an impermeable substance such as oil or paint. By this coating air and water cannot come in direct contact with the surface of the metal. By polishing metallic restorations like amalgam and cast metal to minimize corrosion. The patient should maintain good oral hygiene. Dissimilar metallic restorations should be avoided.

n Passivation Certain metals develop a thin, highly protective oxide film by the reaction with the environment; such a metal is said to be passive. This formation of a protective film by a reactive substance 'is called "passivation". Three metals which are well known for their passivation potential are aluminum. chromium and titanium. Chromium forms a coherent, uniform and nonporous layer of chromic oxide (Cr 203) on their suface after exposure to atmospheric oxygen. This film is self limiting beca. Use it acts as a barrier between oxygen and metal ions necessary for futher corrosion. Passive alloys, such as those containing chromium, are widely used in dentistry. They must contain a minimum of 12 percent chromium.

n Electroplating Electrodeposition or electroplating, the impression to be coated is rnadethe cathode of an electrolytic cell. The anode is composed of the metal to be deposited onto the cathode. surface. The electrolytic solution contains cations of the metal to be deposited. When current is passed through such a cell the anode corrodes, supplying cations, which are transported through the electrolyte to the cathode. Iron, steel and certain other metals that are subjected to corrosion may be electroplated with nickel followed by chromium for protective and esthetic purpose. Copper and silver plating techniques are used in dental laboratories.

 Ideal properties of dental materials
Ideal properties of dental materials Auxiliary dental materials definition
Auxiliary dental materials definition Lesson outline physical properties lesson 2
Lesson outline physical properties lesson 2 Grade 7 ns term 2
Grade 7 ns term 2 Chemical and physical properties
Chemical and physical properties What are preventive dental material?
What are preventive dental material? Polymeric dental materials
Polymeric dental materials Elastic range
Elastic range Auxiliary dental materials examples
Auxiliary dental materials examples Biocompatibility of dental materials
Biocompatibility of dental materials Crown lengthening procedure steps ppt
Crown lengthening procedure steps ppt Auxiliary dental materials
Auxiliary dental materials Introduction to dental materials
Introduction to dental materials Materials department
Materials department What is a quote sandwich
What is a quote sandwich Tensile strength of amalgam
Tensile strength of amalgam Natural materials and man made materials
Natural materials and man made materials 10 useful and harmful materials
10 useful and harmful materials Man made map
Man made map What is adopting materials
What is adopting materials Direct materials budget with multiple materials
Direct materials budget with multiple materials Lesson outline lesson 2 wave properties answer key
Lesson outline lesson 2 wave properties answer key General properties of smart and modern materials
General properties of smart and modern materials Mechanical properties of materials physics
Mechanical properties of materials physics Malleability and ductility
Malleability and ductility Hardness of nanomaterials
Hardness of nanomaterials Properties of materials examples
Properties of materials examples Properties of materials vocabulary
Properties of materials vocabulary Manufacturing property of a material
Manufacturing property of a material Manufacturing properties of materials
Manufacturing properties of materials Optical properties of engineering materials
Optical properties of engineering materials What is the engineering materials
What is the engineering materials Properties of cartridge paper
Properties of cartridge paper What is matter in science grade 7
What is matter in science grade 7 Technology grade 7 term 3 notes
Technology grade 7 term 3 notes Properties of materials examples
Properties of materials examples Difference between smart and modern materials
Difference between smart and modern materials 5 properties of materials
5 properties of materials The total exterior of a business includes the entranceways
The total exterior of a business includes the entranceways Lesson 3 physical and chemical changes answers
Lesson 3 physical and chemical changes answers Outline physical map of africa
Outline physical map of africa The physical outline of a display
The physical outline of a display Extensive vs intensive properties
Extensive vs intensive properties Amino acid optical isomers
Amino acid optical isomers Bromine test for unsaturation
Bromine test for unsaturation Physical properties of sulphuric acid
Physical properties of sulphuric acid 2 hydrogen 1 oxygen
2 hydrogen 1 oxygen Ethan is observing chemical and physical properties
Ethan is observing chemical and physical properties Physical properties of starch
Physical properties of starch Physical properties of ocean water
Physical properties of ocean water Cardboard mechanical properties
Cardboard mechanical properties Physical change
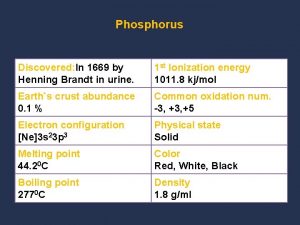
Physical change Chemical properties of phosphorus
Chemical properties of phosphorus Physical properties of paint
Physical properties of paint The physical properties of metals include luster and
The physical properties of metals include luster and Malleability defintion
Malleability defintion Metal heat expansion chart
Metal heat expansion chart Physical properties of protein
Physical properties of protein Protein solubility
Protein solubility Pumice crystal size
Pumice crystal size Don't gamble with physical properties for simulations
Don't gamble with physical properties for simulations