Physical properties of alkanes mostly even Steven Boiling
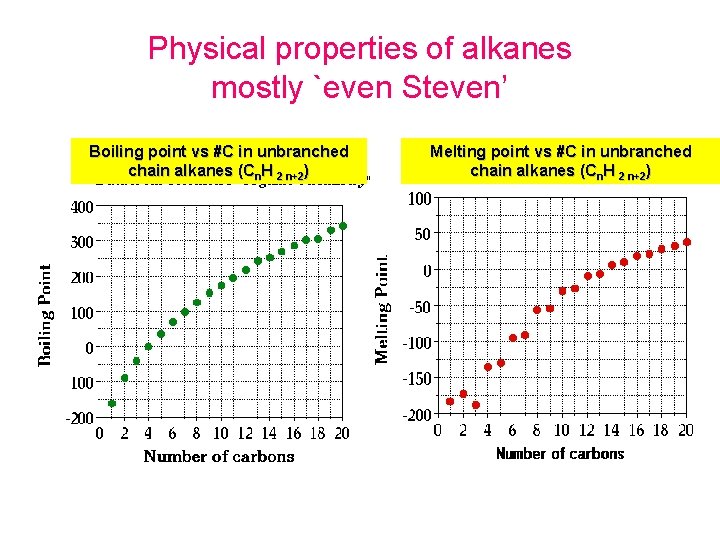
Physical properties of alkanes mostly `even Steven’ Boiling point vs #C in unbranched chain alkanes (Cn. H 2 n+2) Melting point vs #C in unbranched chain alkanes (Cn. H 2 n+2)
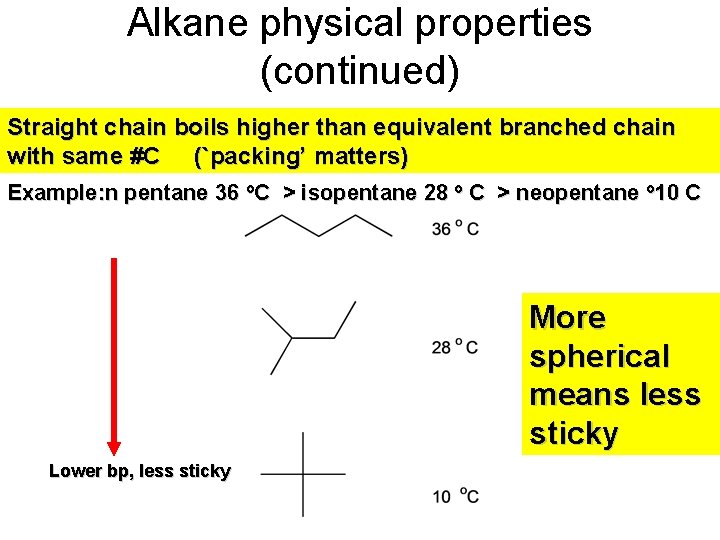
Alkane physical properties (continued) Straight chain boils higher than equivalent branched chain with same #C (`packing’ matters) Example: n pentane 36 o. C > isopentane 28 o C > neopentane o 10 C More spherical means less sticky Lower bp, less sticky
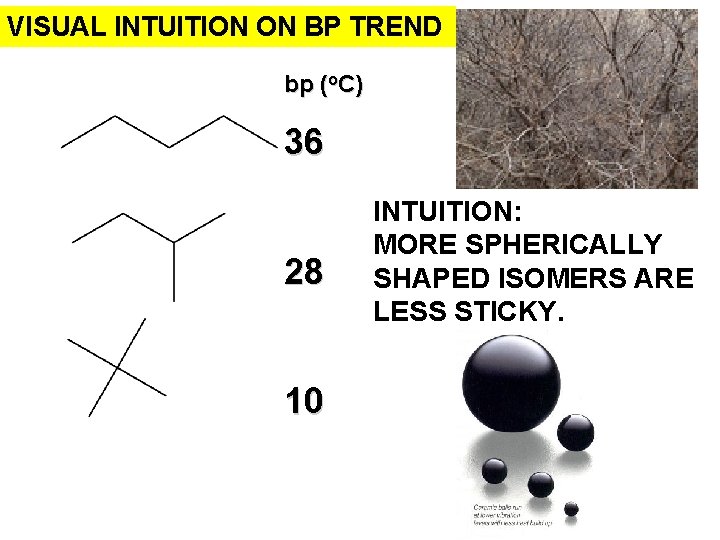
VISUAL INTUITION ON BP TREND bp (o. C) 36 28 10 INTUITION: MORE SPHERICALLY SHAPED ISOMERS ARE LESS STICKY.
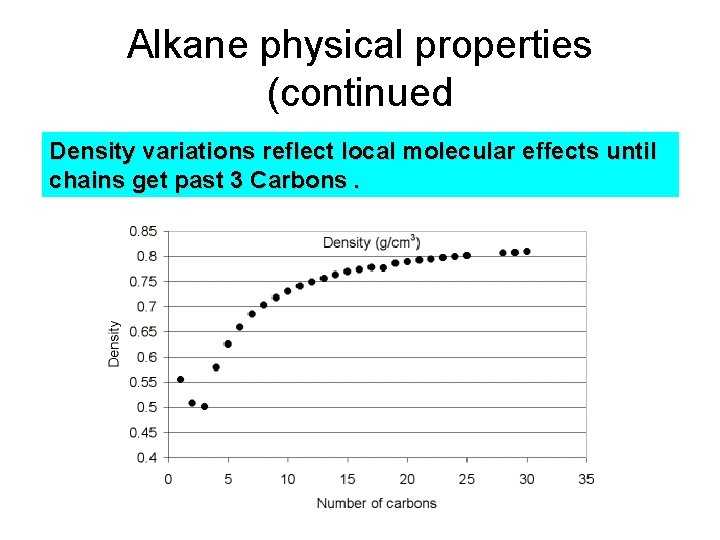
Alkane physical properties (continued Density variations reflect local molecular effects until chains get past 3 Carbons.
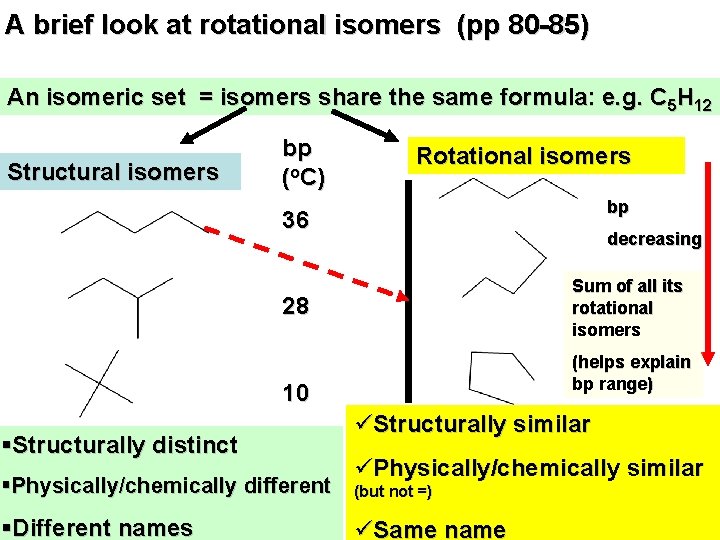
A brief look at rotational isomers (pp 80 -85) An isomeric set = isomers share the same formula: e. g. C 5 H 12 Structural isomers bp (o. C) Rotational isomers bp 36 decreasing Sum of all its rotational isomers 28 (helps explain bp range) 10 §Structurally distinct §Physically/chemically different §Different names üStructurally similar üPhysically/chemically similar (but not =) üSame name
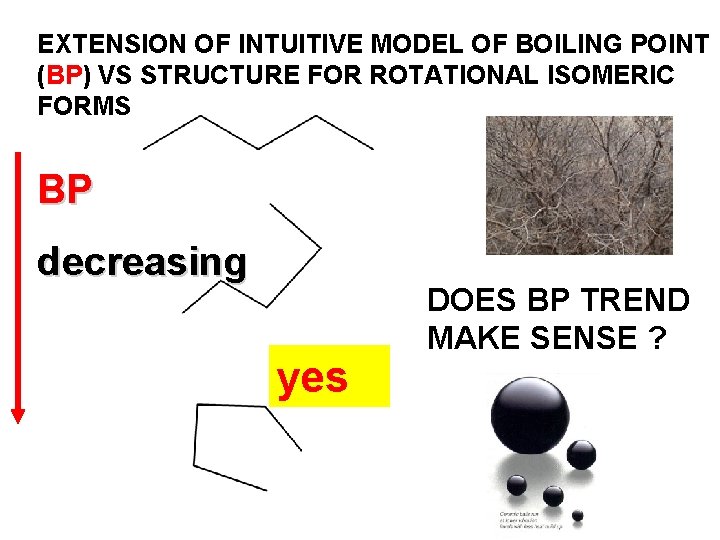
EXTENSION OF INTUITIVE MODEL OF BOILING POINT (BP) VS STRUCTURE FOR ROTATIONAL ISOMERIC FORMS BP decreasing yes DOES BP TREND MAKE SENSE ?
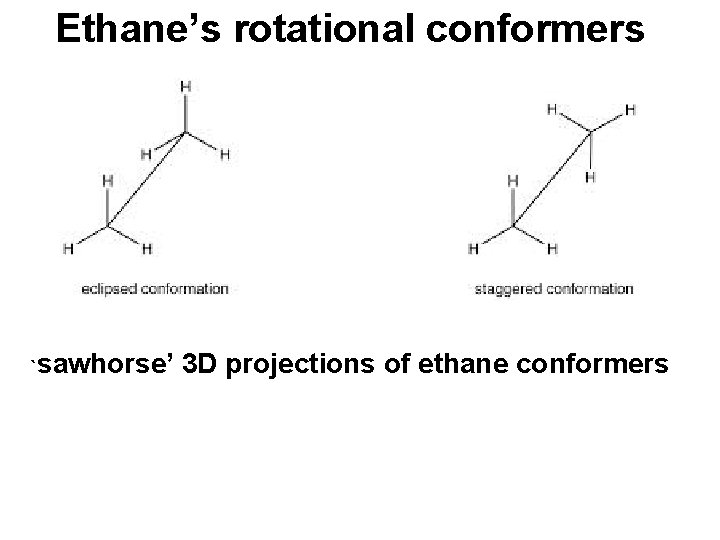
Ethane’s rotational conformers `sawhorse’ 3 D projections of ethane conformers
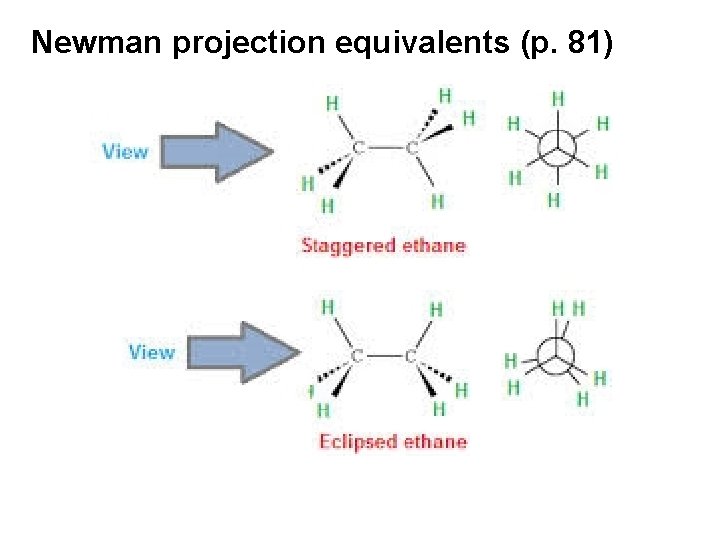
Newman projection equivalents (p. 81)
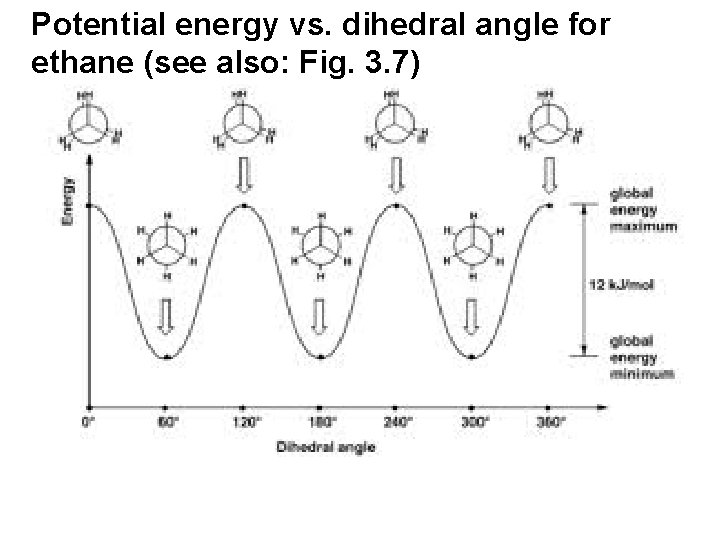
Potential energy vs. dihedral angle for ethane (see also: Fig. 3. 7)
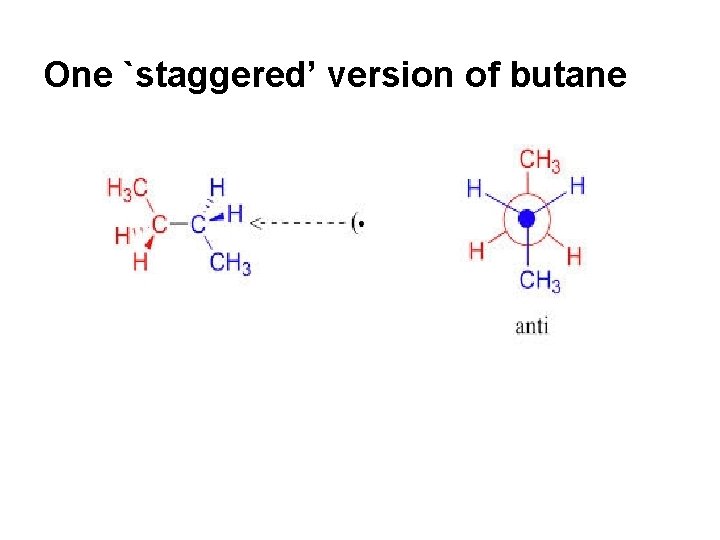
One `staggered’ version of butane
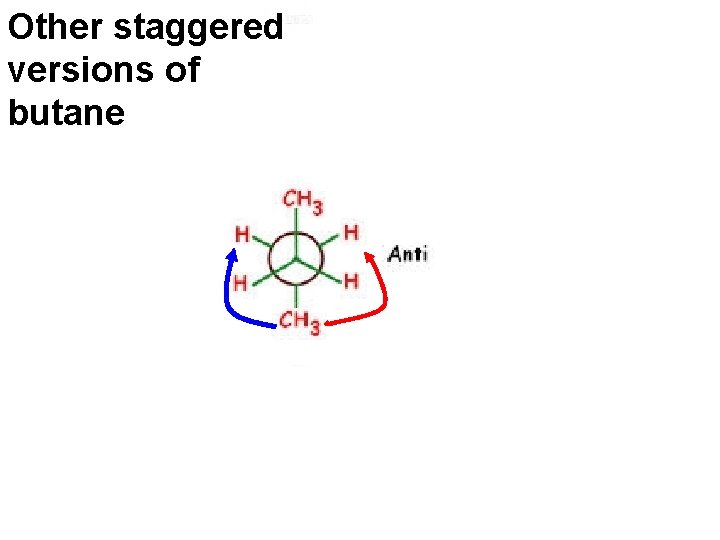
Other staggered versions of butane
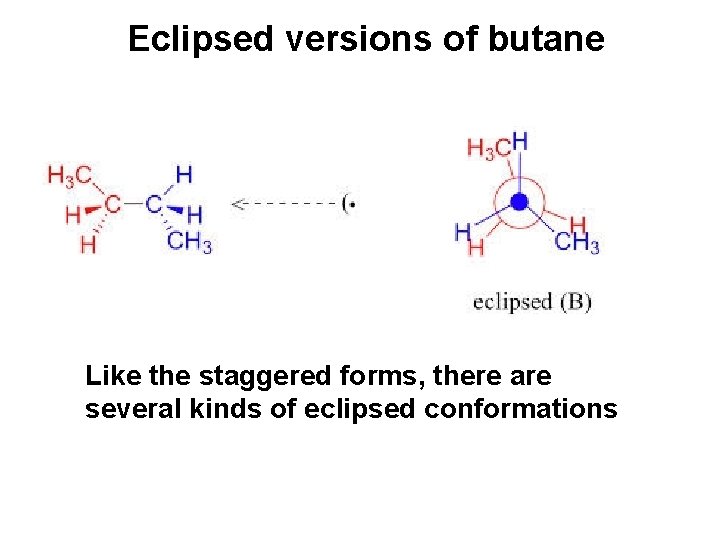
Eclipsed versions of butane Like the staggered forms, there are several kinds of eclipsed conformations
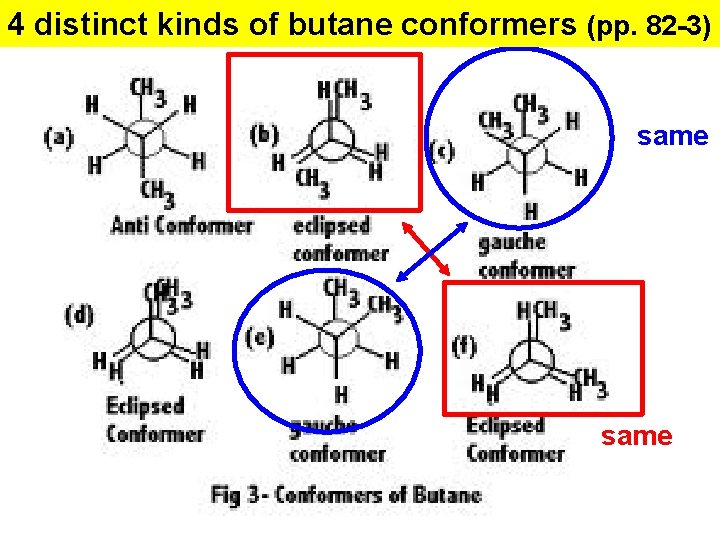
4 distinct kinds of butane conformers (pp. 82 -3) same
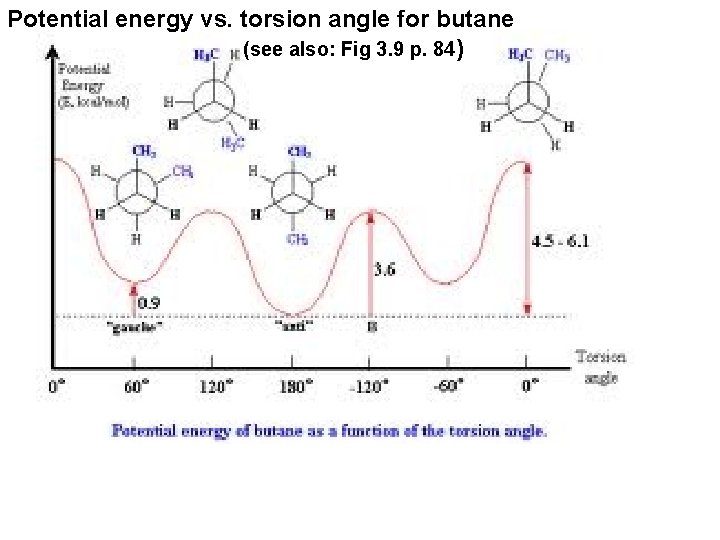
Potential energy vs. torsion angle for butane (see also: Fig 3. 9 p. 84)
- Slides: 14