PHYSICAL PROPERTIES OF ALKANES and ALKENES SAME TREND










- Slides: 61

PHYSICAL PROPERTIES OF ALKANES and ALKENES SAME TREND AND REASON FOR BOTH “ANES” AND “ENES” ALL NON POLAR AND HAVE WEAK VAN DER WALLS FORCES Melting point general increase with molecular mass the trend is not as regular as that for boiling point. Solubilityalkanes are non-polar so are immiscible with water they are soluble in most organic solvents.

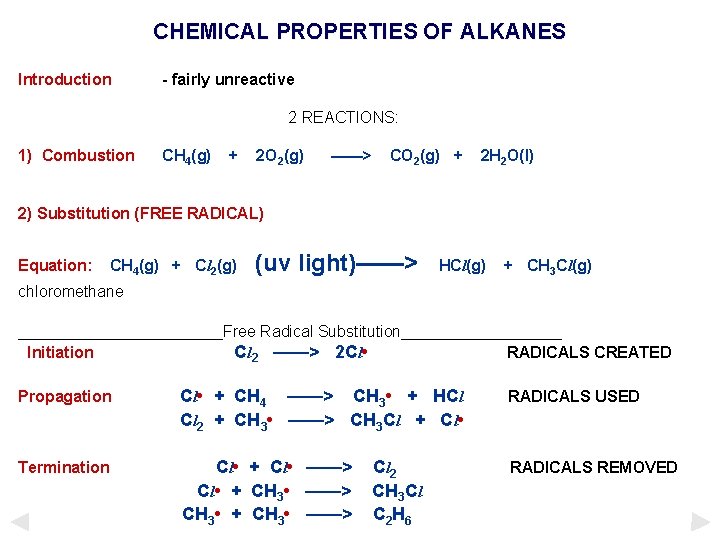
CHEMICAL PROPERTIES OF ALKANES Introduction - fairly unreactive 2 REACTIONS: 1) Combustion CH 4(g) + 2 O 2(g) ——> CO 2(g) + 2 H 2 O(l) 2) Substitution (FREE RADICAL) Equation: CH 4(g) + Cl 2(g) (uv light)——> HCl(g) + CH 3 Cl(g) chloromethane ____________Free Radical Substitution_________ Initiation Cl 2 ——> 2 Cl • RADICALS CREATED Propagation Cl • + CH 4 ——> CH 3 • + HCl Cl 2 + CH 3 • ——> CH 3 Cl + Cl • RADICALS USED Termination Cl • + Cl • ——> Cl • + CH 3 • ——> CH 3 • + CH 3 • ——> RADICALS REMOVED C l 2 CH 3 Cl C 2 H 6

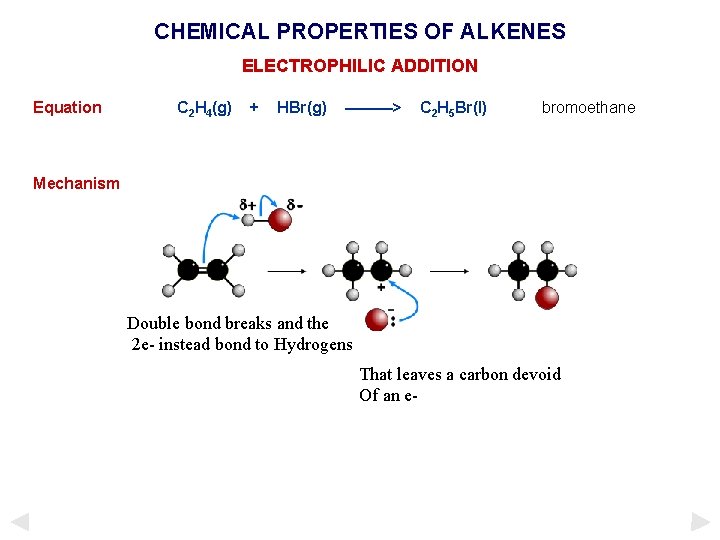
CHEMICAL PROPERTIES OF ALKENES ELECTROPHILIC ADDITION Equation C 2 H 4(g) + HBr(g) ———> C 2 H 5 Br(l) bromoethane Mechanism Double bond breaks and the 2 e- instead bond to Hydrogens That leaves a carbon devoid Of an e-

CHEMICAL PROPERTIES OF ALKENES ELECTROPHILIC ADDITION OF HYDROGEN BROMIDE ANIMATED MECHANISM Animation repeats continuously after every 10 seconds

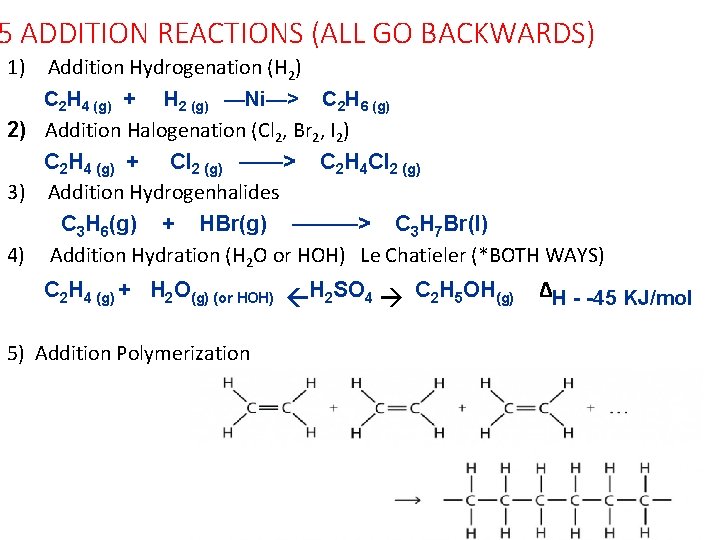
5 ADDITION REACTIONS (ALL GO BACKWARDS) 1) Addition Hydrogenation (H 2) C 2 H 4 (g) + H 2 (g) —Ni—> C 2 H 6 (g) 2) Addition Halogenation (Cl 2, Br 2, I 2) C 2 H 4 (g) + Cl 2 (g) ——> C 2 H 4 Cl 2 (g) 3) Addition Hydrogenhalides C 3 H 6(g) + HBr(g) ———> C 3 H 7 Br(l) 4) Addition Hydration (H 2 O or HOH) Le Chatieler (*BOTH WAYS) C 2 H 4 (g) + H 2 O(g) (or HOH) H 2 SO 4 C 2 H 5 OH(g) 5) Addition Polymerization ΔH - -45 KJ/mol

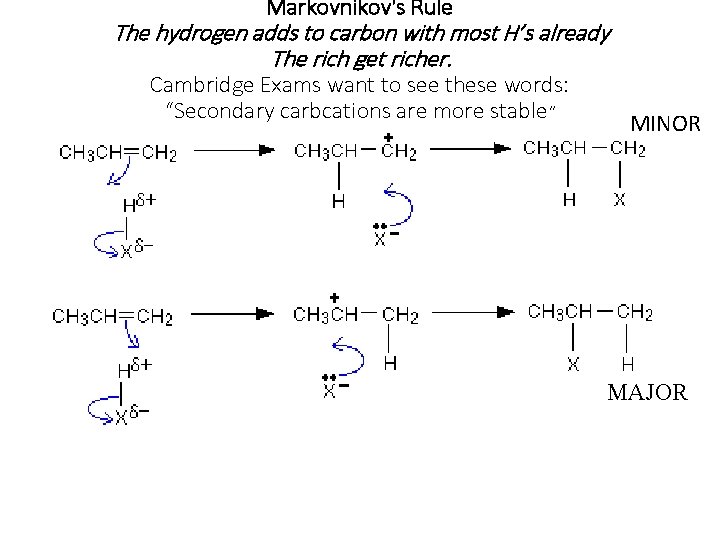
Markovnikov's Rule The hydrogen adds to carbon with most H’s already The rich get richer. Cambridge Exams want to see these words: “Secondary carbcations are more stable” MINOR MAJOR

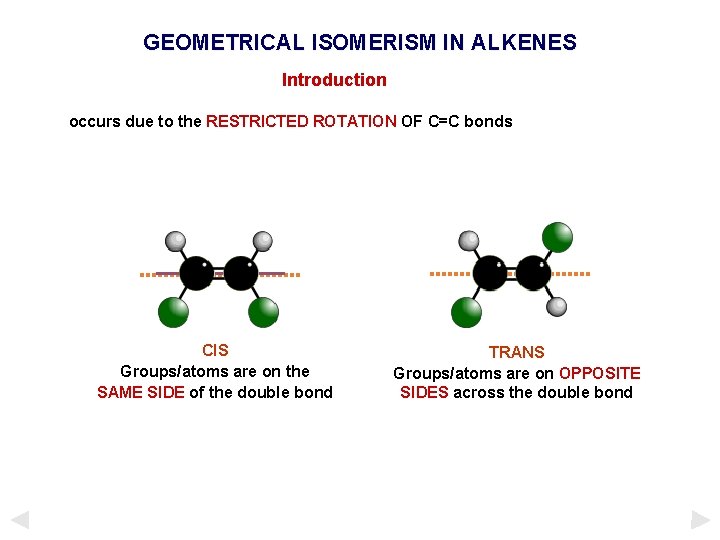
GEOMETRICAL ISOMERISM IN ALKENES Introduction occurs due to the RESTRICTED ROTATION OF C=C bonds CIS Groups/atoms are on the SAME SIDE of the double bond TRANS Groups/atoms are on OPPOSITE SIDES across the double bond

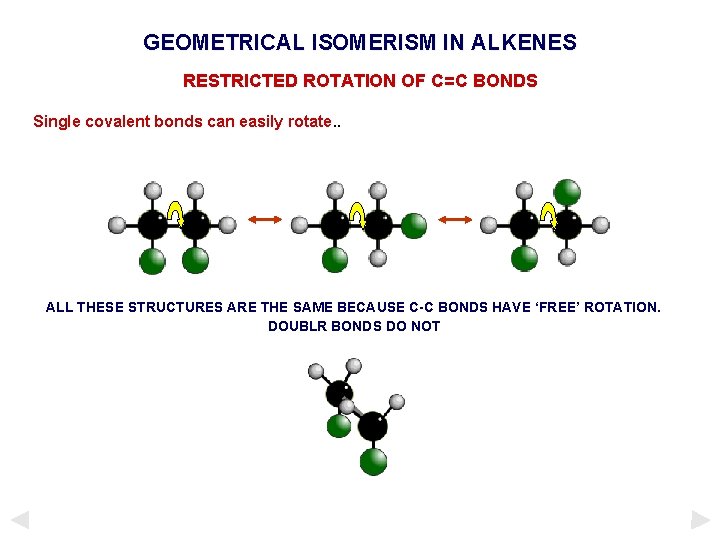
GEOMETRICAL ISOMERISM IN ALKENES RESTRICTED ROTATION OF C=C BONDS Single covalent bonds can easily rotate. . ALL THESE STRUCTURES ARE THE SAME BECAUSE C-C BONDS HAVE ‘FREE’ ROTATION. DOUBLR BONDS DO NOT

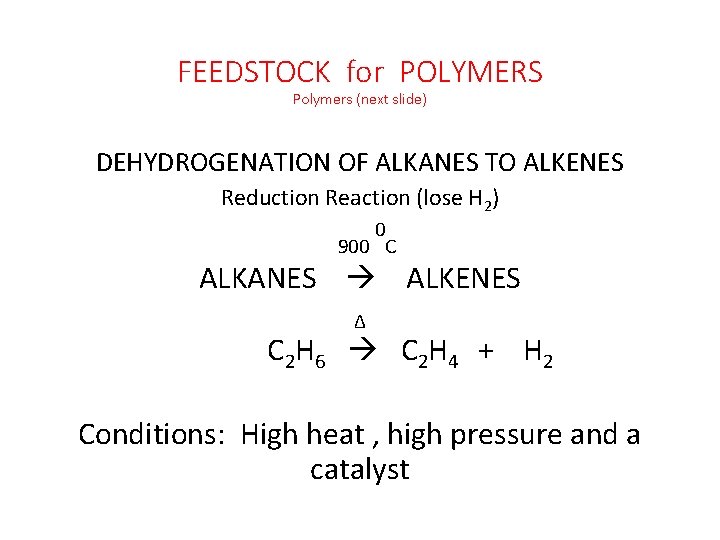
FEEDSTOCK for POLYMERS Polymers (next slide) DEHYDROGENATION OF ALKANES TO ALKENES Reduction Reaction (lose H 2) 0 900 C ALKANES ALKENES ∆ C 2 H 6 C 2 H 4 + H 2 Conditions: High heat , high pressure and a catalyst

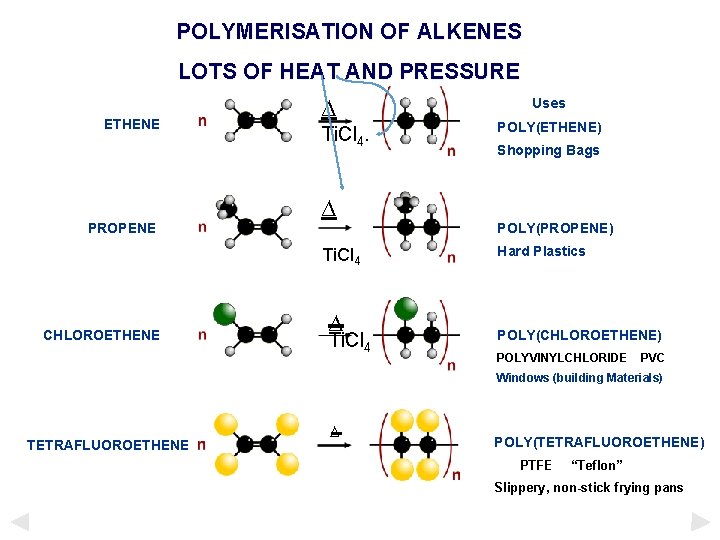
POLYMERISATION OF ALKENES LOTS OF HEAT AND PRESSURE ETHENE PROPENE ∆ Ti. Cl 4 CHLOROETHENE ∆ Ti. Cl 4 Uses POLY(ETHENE) Shopping Bags POLY(PROPENE) Hard Plastics POLY(CHLOROETHENE) POLYVINYLCHLORIDE PVC Windows (building Materials) TETRAFLUOROETHENE ∆ POLY(TETRAFLUOROETHENE) PTFE “Teflon” Slippery, non-stick frying pans

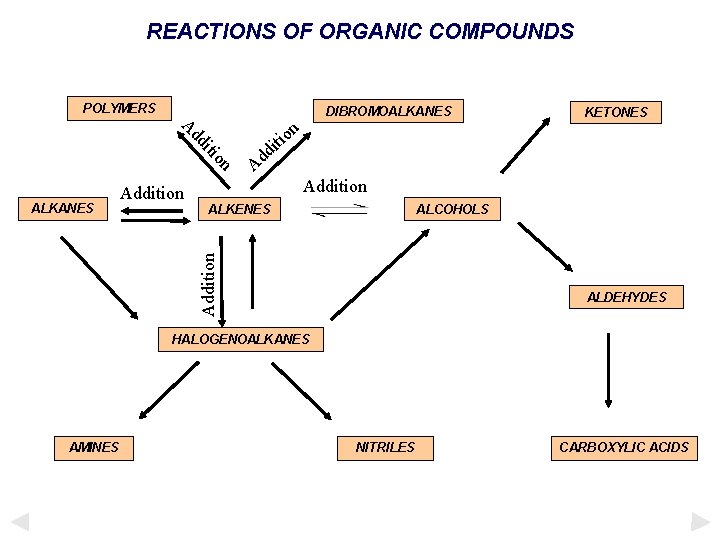
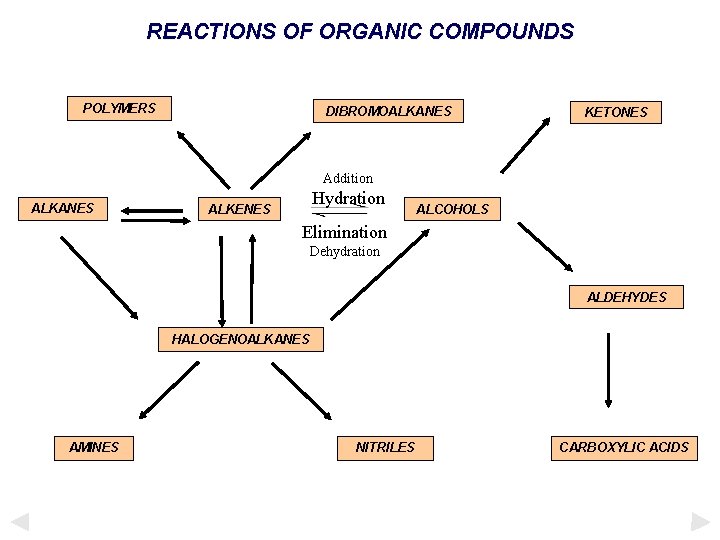
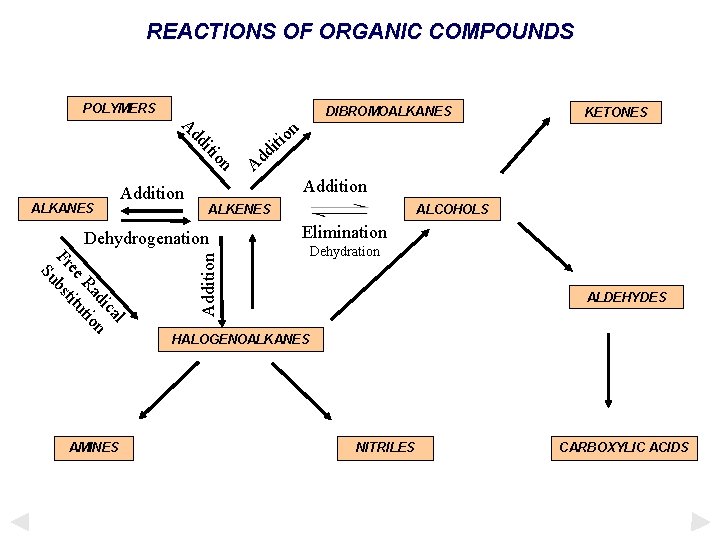
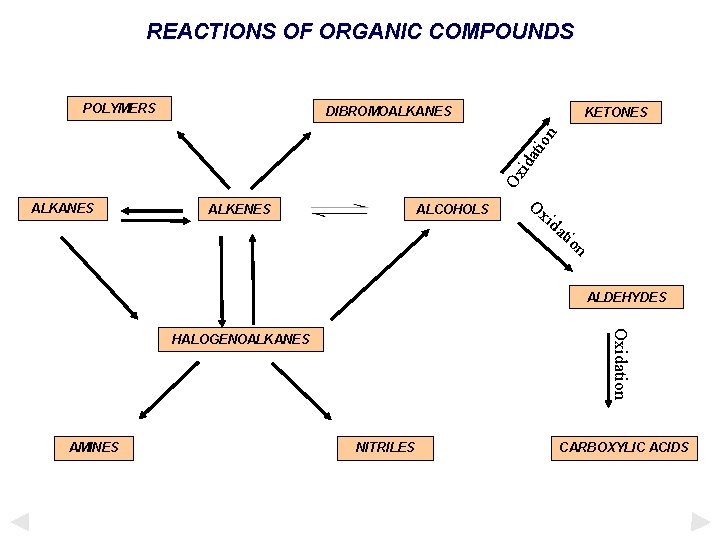
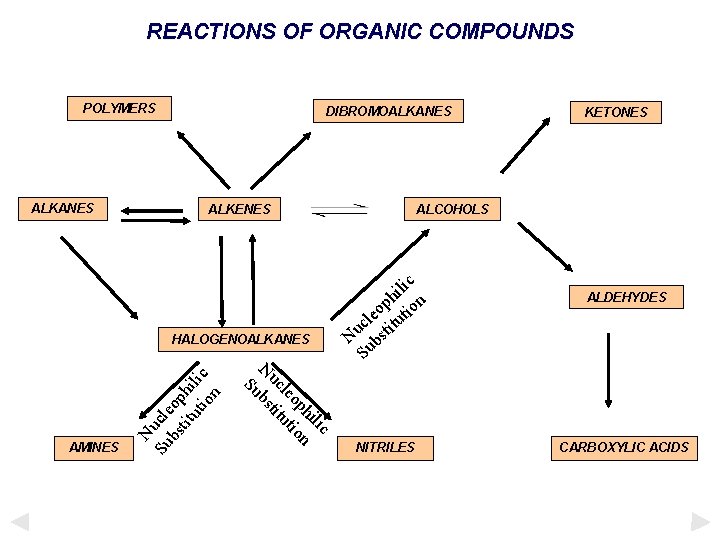
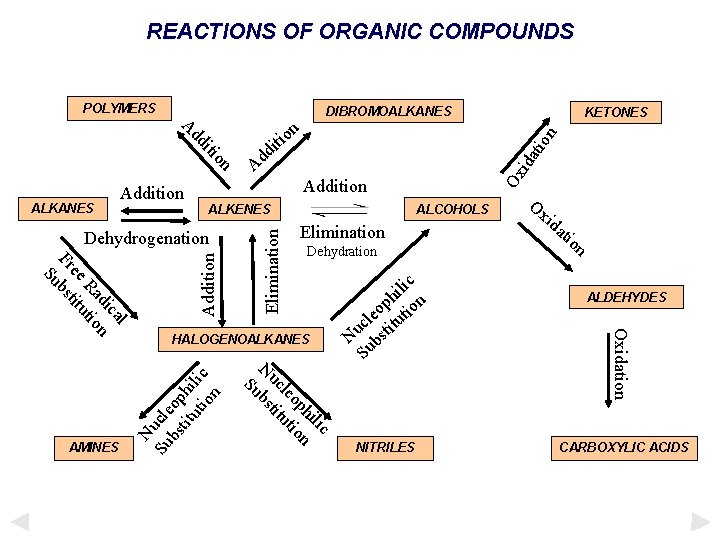
REACTIONS OF ORGANIC COMPOUNDS POLYMERS DIBROMOALKANES n o iti on iti dd A d d A Addition ALKENES ALCOHOLS Addition ALKANES Addition KETONES ALDEHYDES HALOGENOALKANES AMINES NITRILES CARBOXYLIC ACIDS

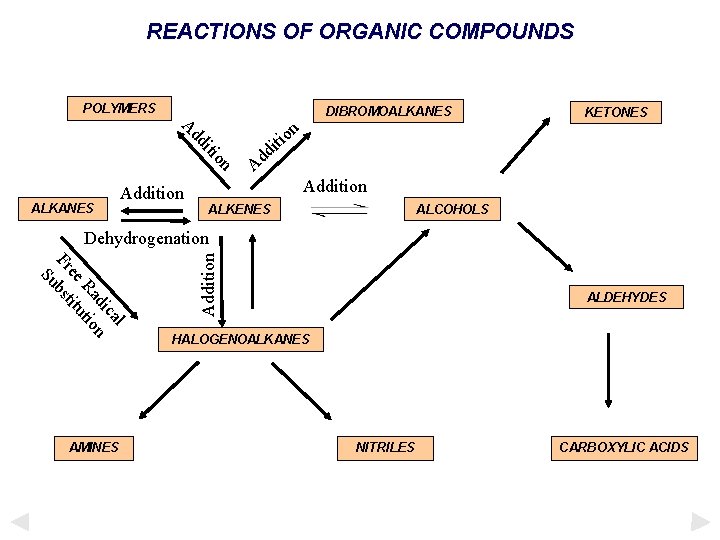
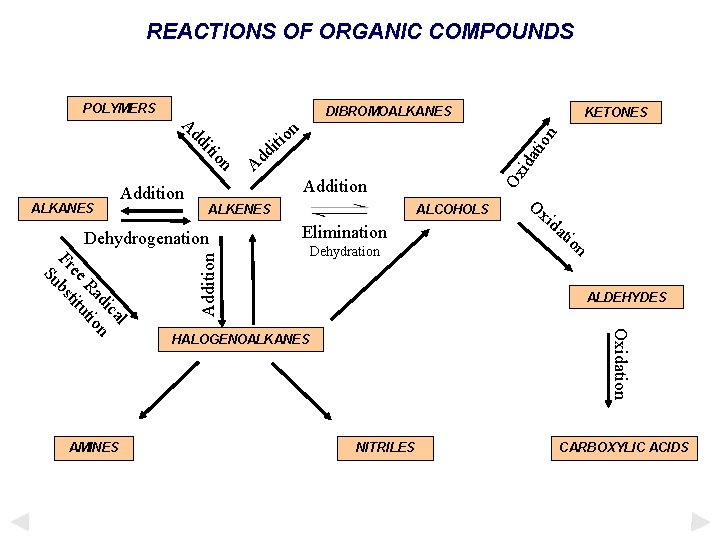
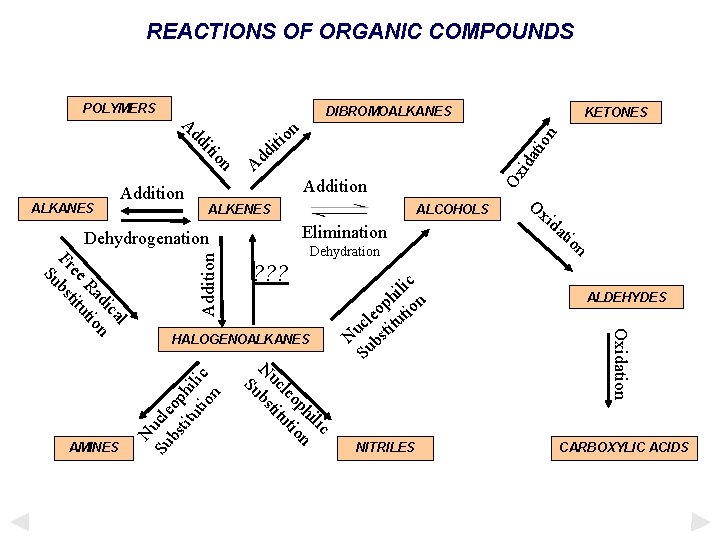
REACTIONS OF ORGANIC COMPOUNDS POLYMERS DIBROMOALKANES n o iti on iti dd A ALKANES Addition KETONES d d A Addition ALKENES ALCOHOLS l ca di n Ra tio ee itu Fr bst Su AMINES Addition Dehydrogenation ALDEHYDES HALOGENOALKANES NITRILES CARBOXYLIC ACIDS

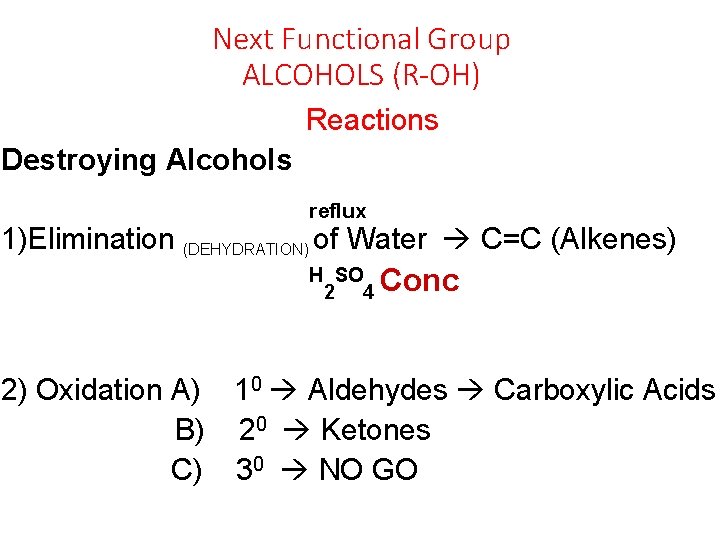
Next Functional Group ALCOHOLS (R-OH) Reactions Destroying Alcohols reflux 1)Elimination (DEHYDRATION) of Water C=C (Alkenes) H SO Conc 2 4 2) Oxidation A) 10 Aldehydes Carboxylic Acids B) 20 Ketones C) 30 NO GO

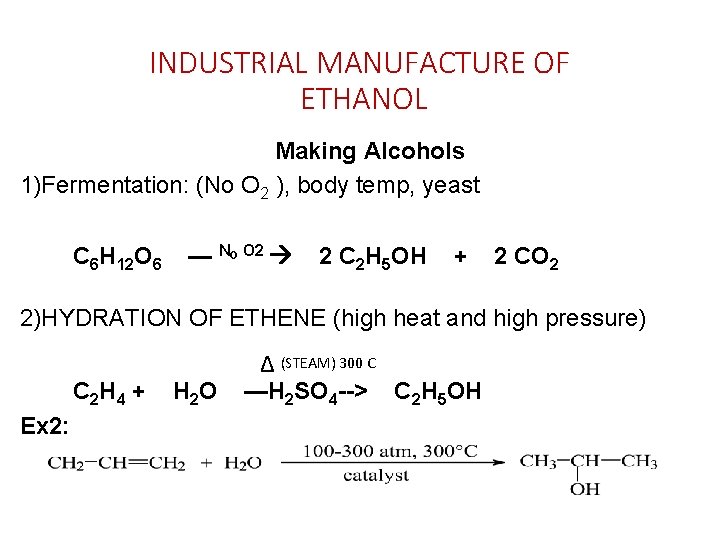
INDUSTRIAL MANUFACTURE OF ETHANOL Making Alcohols 1)Fermentation: (No O 2 ), body temp, yeast C 6 H 12 O 6 — NO O 2 2 C 2 H 5 OH + 2 CO 2 2)HYDRATION OF ETHENE (high heat and high pressure) C 2 H 4 + Ex 2: H 2 O ∆ (STEAM) 300 C —H 2 SO 4 --> C 2 H 5 OH

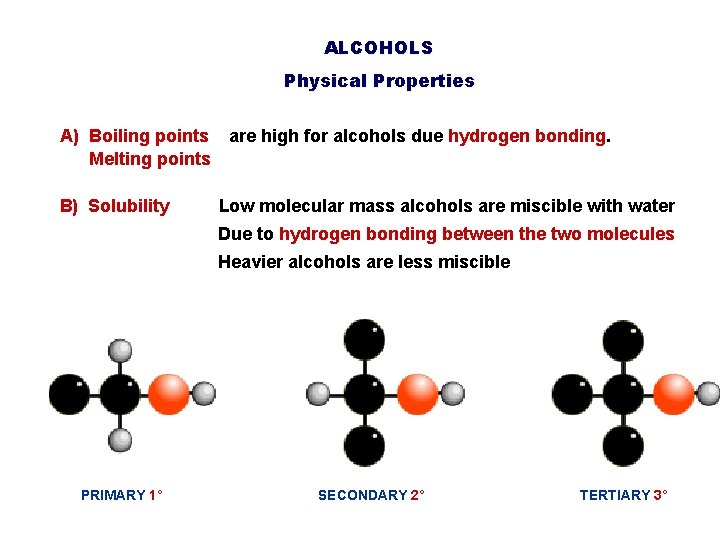
ALCOHOLS Physical Properties A) Boiling points are high for alcohols due hydrogen bonding. Melting points B) Solubility Low molecular mass alcohols are miscible with water Due to hydrogen bonding between the two molecules Heavier alcohols are less miscible PRIMARY 1° SECONDARY 2° TERTIARY 3°

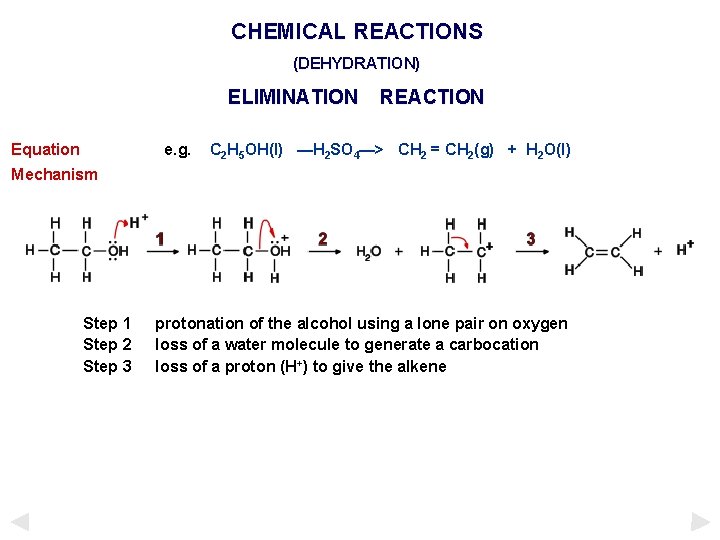
CHEMICAL REACTIONS (DEHYDRATION) ELIMINATION Equation e. g. REACTION C 2 H 5 OH(l) —H 2 SO 4—> CH 2 = CH 2(g) + H 2 O(l) Mechanism Step 1 Step 2 Step 3 protonation of the alcohol using a lone pair on oxygen loss of a water molecule to generate a carbocation loss of a proton (H+) to give the alkene

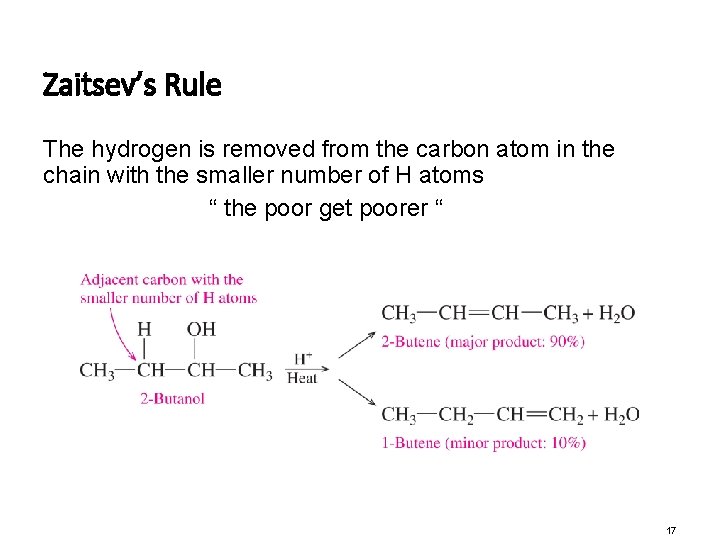
Zaitsev’s Rule The hydrogen is removed from the carbon atom in the chain with the smaller number of H atoms “ the poor get poorer “ 17

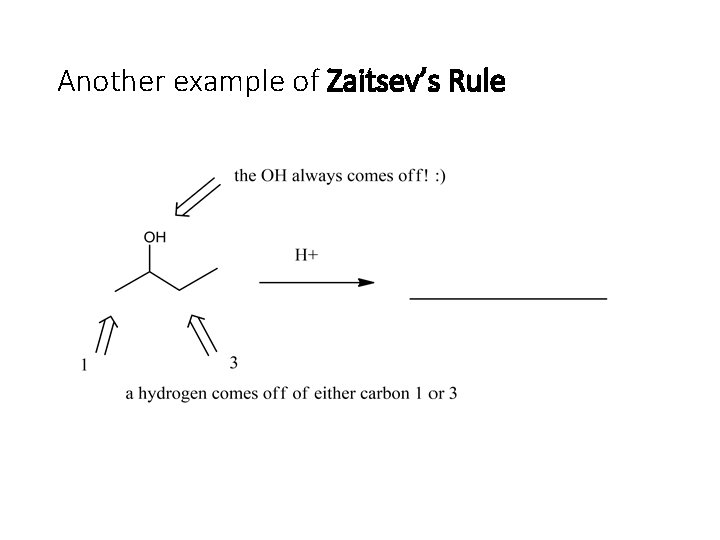
Another example of Zaitsev’s Rule

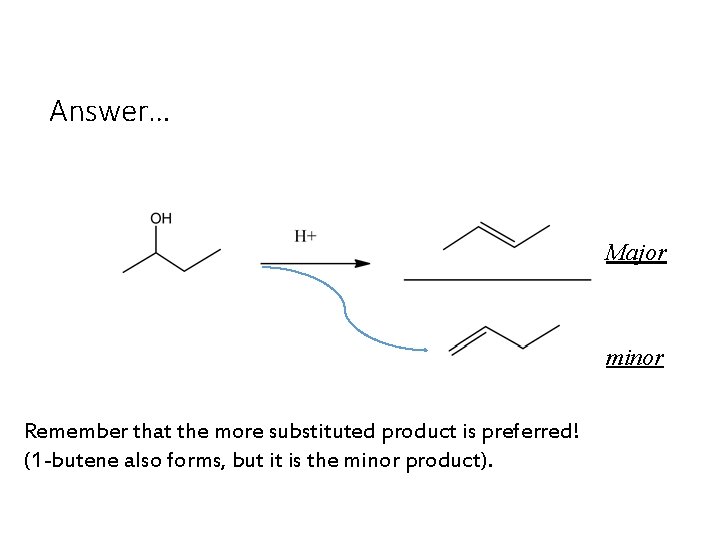
Answer… Major minor Remember that the more substituted product is preferred! (1 -butene also forms, but it is the minor product).

This one, then one more… 20

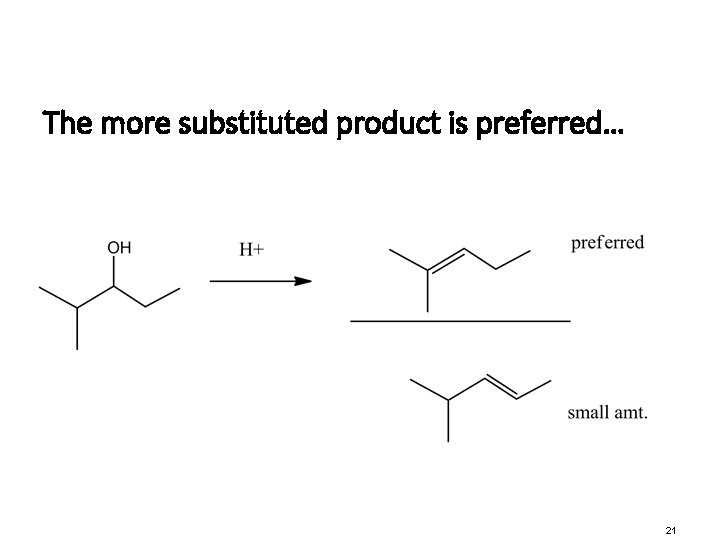
The more substituted product is preferred… 21

REACTIONS OF ORGANIC COMPOUNDS POLYMERS DIBROMOALKANES KETONES Addition ALKANES Hydration ALKENES ALCOHOLS Elimination Dehydration ALDEHYDES HALOGENOALKANES AMINES NITRILES CARBOXYLIC ACIDS

REACTIONS OF ORGANIC COMPOUNDS POLYMERS DIBROMOALKANES n o iti on iti dd A ALKANES Addition l ca di n Ra tio ee itu Fr bst Su AMINES d d A Addition ALKENES Dehydrogenation KETONES ALCOHOLS Elimination Dehydration ALDEHYDES HALOGENOALKANES NITRILES CARBOXYLIC ACIDS

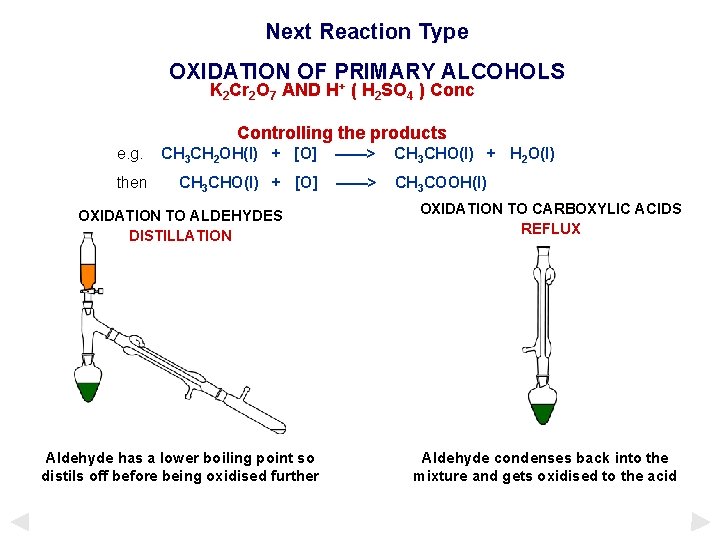
Next Reaction Type OXIDATION OF PRIMARY ALCOHOLS + K 2 Cr 2 O 7 AND H ( H 2 SO 4 ) Conc Controlling the products e. g. CH 3 CH 2 OH(l) + [O] ——> CH 3 CHO(l) + H 2 O(l) then CH 3 CHO(l) + [O] ——> CH 3 COOH(l) OXIDATION TO ALDEHYDES DISTILLATION Aldehyde has a lower boiling point so distils off before being oxidised further OXIDATION TO CARBOXYLIC ACIDS REFLUX Aldehyde condenses back into the mixture and gets oxidised to the acid

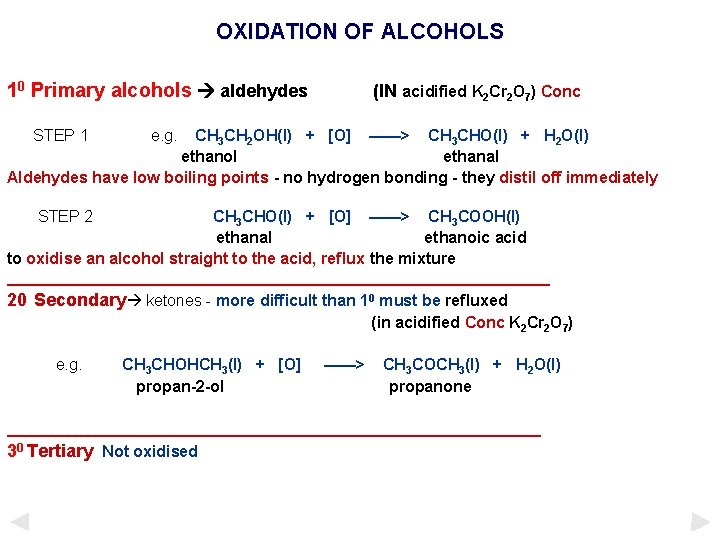
OXIDATION OF ALCOHOLS 10 Primary alcohols aldehydes STEP 1 (IN acidified K 2 Cr 2 O 7) Conc e. g. CH 3 CH 2 OH(l) + [O] ——> CH 3 CHO(l) + H 2 O(l) ethanol ethanal Aldehydes have low boiling points - no hydrogen bonding - they distil off immediately STEP 2 CH 3 CHO(l) + [O] ——> CH 3 COOH(l) ethanal ethanoic acid to oxidise an alcohol straight to the acid, reflux the mixture _______________________________ 20 Secondary ketones - more difficult than 10 must be refluxed (in acidified Conc K 2 Cr 2 O 7) e. g. CH 3 CHOHCH 3(l) + [O] propan-2 -ol ——> CH 3 COCH 3(l) + H 2 O(l) propanone ______________________________ 30 Tertiary Not oxidised

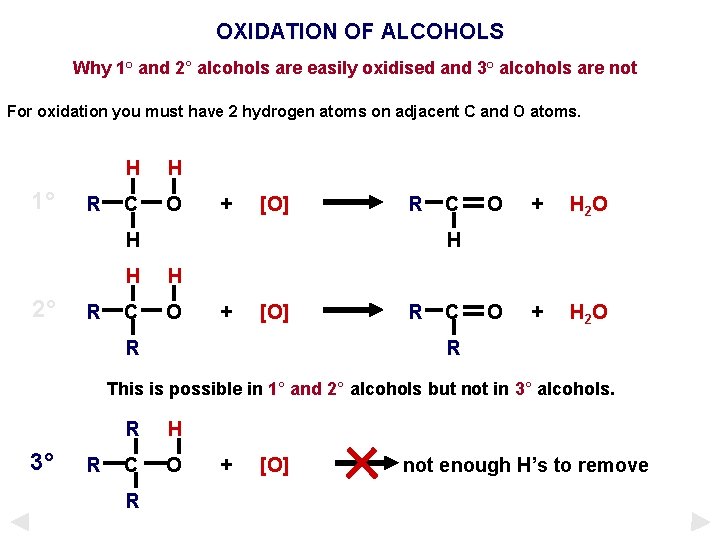
OXIDATION OF ALCOHOLS Why 1° and 2° alcohols are easily oxidised and 3° alcohols are not For oxidation you must have 2 hydrogen atoms on adjacent C and O atoms. 1° R H H C O + [O] R H 2° R C O + H 2 O H H H C O + [O] R R C R This is possible in 1° and 2° alcohols but not in 3° alcohols. 3° R R H C O R + [O] not enough H’s to remove

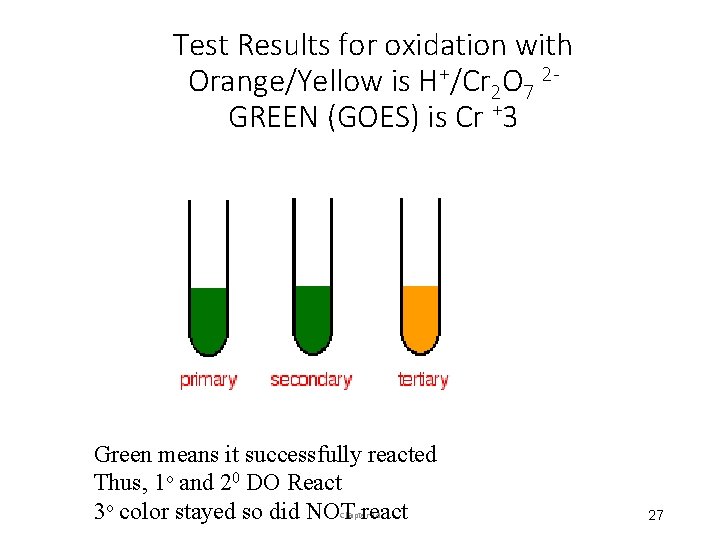
Test Results for oxidation with Orange/Yellow is H+/Cr 2 O 7 2 GREEN (GOES) is Cr +3 Green means it successfully reacted Thus, 1 o and 20 DO React 3 o color stayed so did NOT react Chapter 14 27

REACTIONS OF ORGANIC COMPOUNDS POLYMERS DIBROMOALKANES Ox ida tio n KETONES ALKANES ALKENES ALCOHOLS Ox id ati on ALDEHYDES Oxidation HALOGENOALKANES AMINES NITRILES CARBOXYLIC ACIDS

REACTIONS OF ORGANIC COMPOUNDS POLYMERS DIBROMOALKANES n tio ida ALCOHOLS Elimination Dehydration Ox id ati on ALDEHYDES Oxidation l ca di n Ra tio ee itu Fr bst Su Addition Ox on iti dd Addition ALKENES Dehydrogenation AMINES d d A n o iti A ALKANES Addition KETONES HALOGENOALKANES NITRILES CARBOXYLIC ACIDS

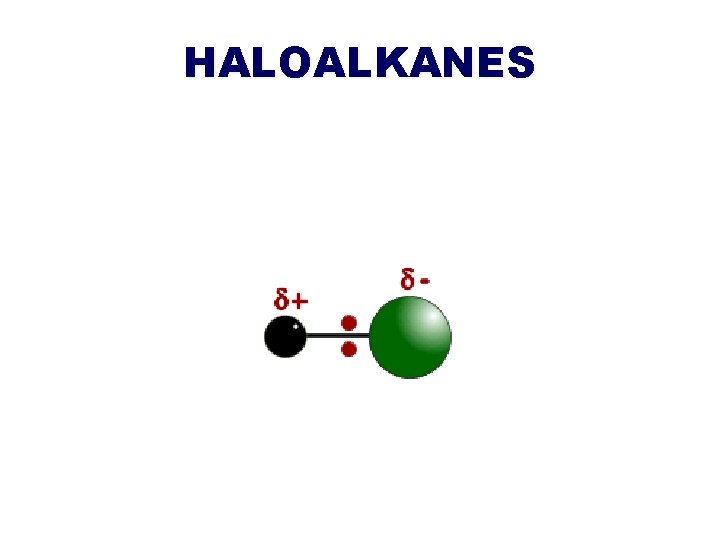
HALOALKANES

Nucleophilic Substitution Swapping Generic Equation: Swap R-X + Nu: R-Nu + : X The problem lies in the mechanism.

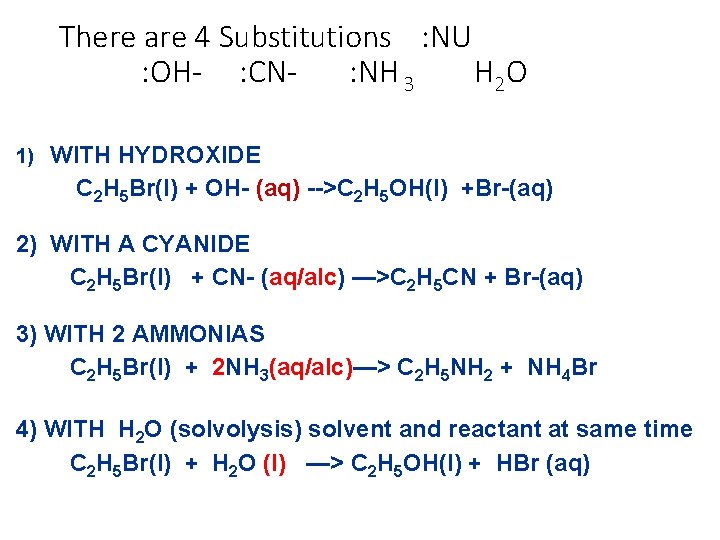
There are 4 Substitutions : NU : OH- : CN: NH 3 H 2 O 1) WITH HYDROXIDE C 2 H 5 Br(l) + OH- (aq) -->C 2 H 5 OH(l) +Br-(aq) 2) WITH A CYANIDE C 2 H 5 Br(l) + CN- (aq/alc) —>C 2 H 5 CN + Br-(aq) 3) WITH 2 AMMONIAS C 2 H 5 Br(l) + 2 NH 3(aq/alc)—> C 2 H 5 NH 2 + NH 4 Br 4) WITH H 2 O (solvolysis) solvent and reactant at same time C 2 H 5 Br(l) + H 2 O (l) —> C 2 H 5 OH(l) + HBr (aq)

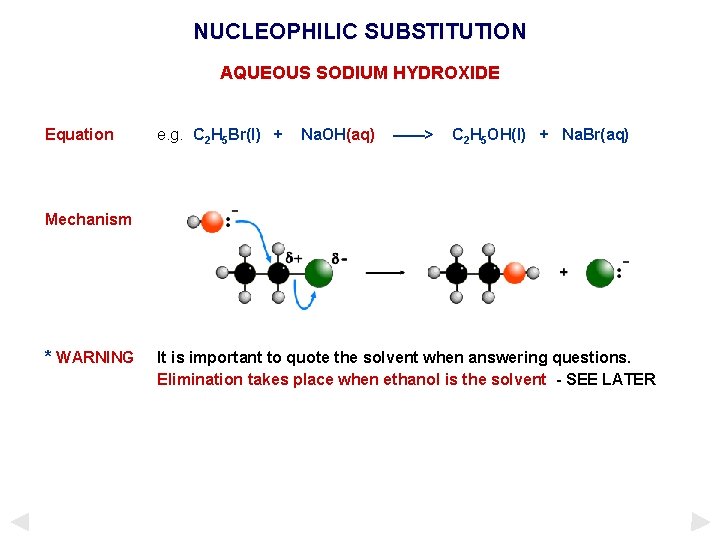
NUCLEOPHILIC SUBSTITUTION AQUEOUS SODIUM HYDROXIDE Equation e. g. C 2 H 5 Br(l) + Na. OH(aq) ——> C 2 H 5 OH(l) + Na. Br(aq) Mechanism * WARNING It is important to quote the solvent when answering questions. Elimination takes place when ethanol is the solvent - SEE LATER

NUCLEOPHILIC SUBSTITUTION AQUEOUS SODIUM HYDROXIDE ANIMATED MECHANISM

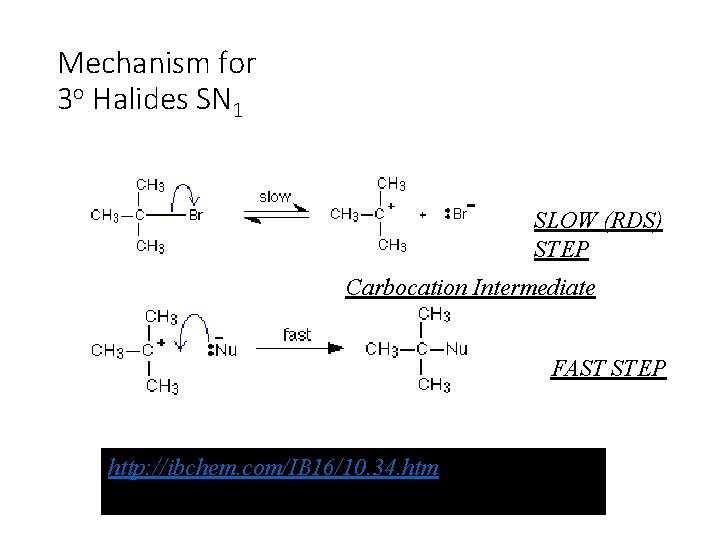
Mechanism for 3 o Halides SN 1 SLOW (RDS) STEP Carbocation Intermediate FAST STEP http: //ibchem. com/IB 16/10. 34. htm

SN 1 Top pic 3 o SN 2 Bottom

REACTIONS OF ORGANIC COMPOUNDS POLYMERS ALKANES DIBROMOALKANES ALKENES Nu lic hi n op o le uti uc tit N bs Su AMINES Su cle bs op tit hi ut lic io n HALOGENOALKANES KETONES ALCOHOLS lic i ph ion o le itut c Nu ubst S NITRILES ALDEHYDES CARBOXYLIC ACIDS

REACTIONS OF ORGANIC COMPOUNDS POLYMERS DIBROMOALKANES n tio ida Addition ALKENES Dehydration ? ? ? Nu lic hi n op o le uti uc tit N bs Su Su cle bs op tit hi ut lic io n HALOGENOALKANES lic i ph ion o le itut c Nu ubst S NITRILES Ox id ati on ALDEHYDES Oxidation l ca di n Ra tio ee itu Fr bst Su Addition ALCOHOLS Elimination Dehydrogenation AMINES Ox on iti dd d d A n o iti A ALKANES Addition KETONES CARBOXYLIC ACIDS

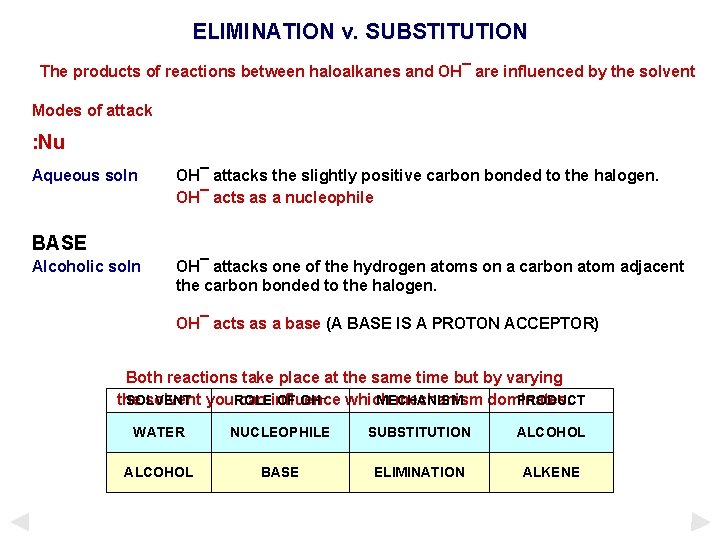
ELIMINATION v. SUBSTITUTION The products of reactions between haloalkanes and OH¯ are influenced by the solvent Modes of attack : Nu Aqueous soln OH¯ attacks the slightly positive carbon bonded to the halogen. OH¯ acts as a nucleophile BASE Alcoholic soln OH¯ attacks one of the hydrogen atoms on a carbon atom adjacent the carbon bonded to the halogen. OH¯ acts as a base (A BASE IS A PROTON ACCEPTOR) Both reactions take place at the same time but by varying the solvent you. ROLE can influence mechanism dominates. SOLVENT OF OH– which MECHANISM PRODUCT WATER NUCLEOPHILE SUBSTITUTION ALCOHOL BASE ELIMINATION ALKENE

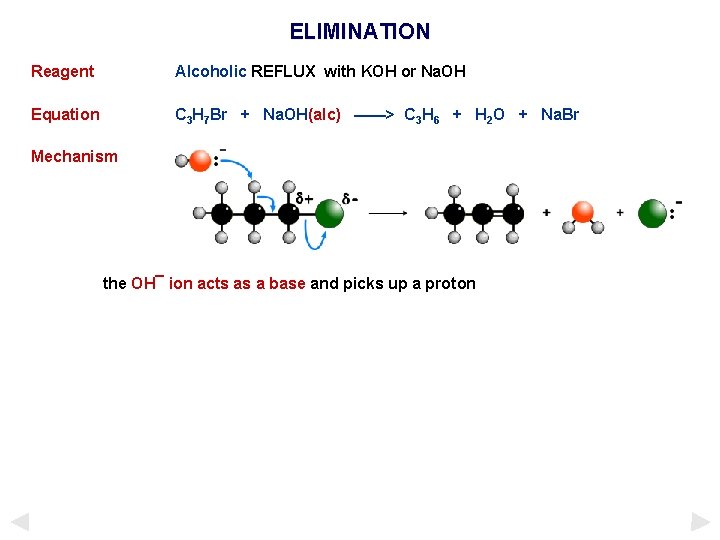
ELIMINATION Reagent Alcoholic REFLUX with KOH or Na. OH Equation C 3 H 7 Br + Na. OH(alc) ——> C 3 H 6 + H 2 O + Na. Br Mechanism the OH¯ ion acts as a base and picks up a proton

ELIMINATION ANIMATED MECHANISM

REACTIONS OF ORGANIC COMPOUNDS POLYMERS DIBROMOALKANES n Elimination tio ida Elimination Dehydration lic hi n op o le uti uc tit N bs Su Su cle bs op tit hi ut lic io n lic i ph ion o le itut c Nu ubst S NITRILES Ox id ati on ALDEHYDES Oxidation l ca di n Ra tio ee itu Fr bst Su Addition ALCOHOLS HALOGENOALKANES Nu Ox on iti dd Addition ALKENES Dehydrogenation AMINES d d A n o iti A ALKANES Addition KETONES CARBOXYLIC ACIDS

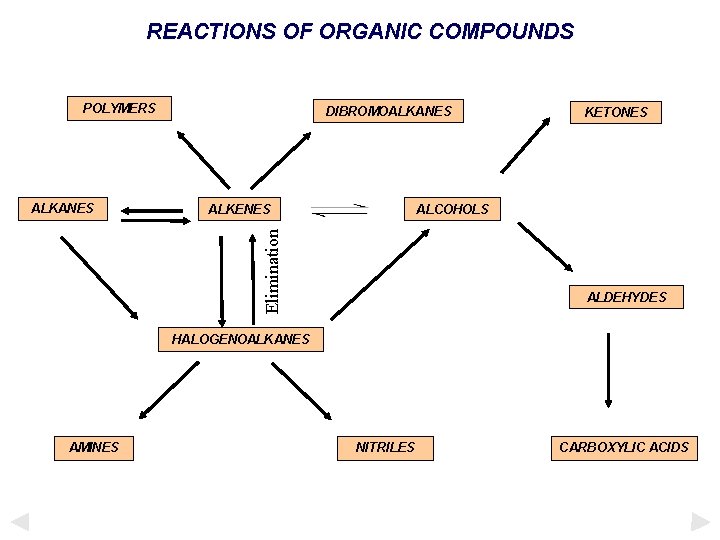
REACTIONS OF ORGANIC COMPOUNDS POLYMERS ALKENES KETONES ALCOHOLS Elimination ALKANES DIBROMOALKANES ALDEHYDES HALOGENOALKANES AMINES NITRILES CARBOXYLIC ACIDS

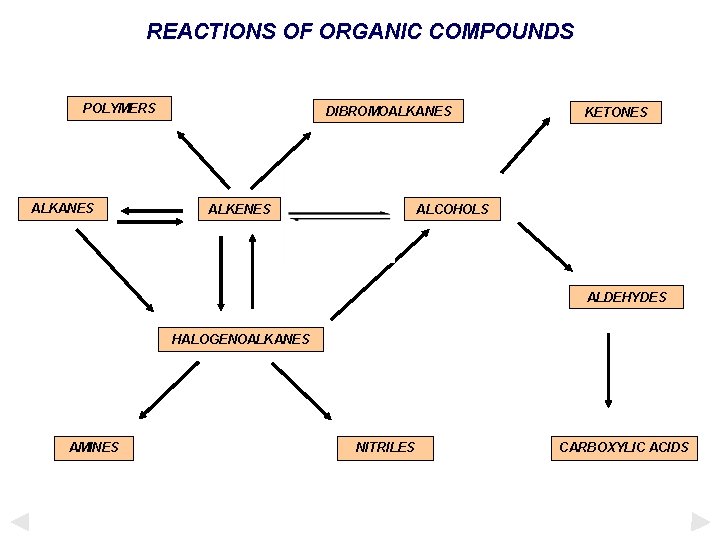
REACTIONS OF ORGANIC COMPOUNDS POLYMERS ALKANES DIBROMOALKANES ALKENES KETONES ALCOHOLS ALDEHYDES HALOGENOALKANES AMINES NITRILES CARBOXYLIC ACIDS

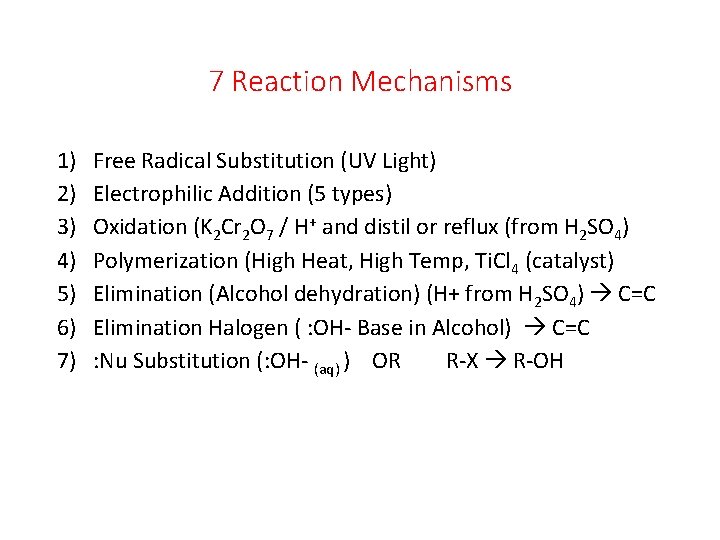
7 Reaction Mechanisms 1) 2) 3) 4) 5) 6) 7) Free Radical Substitution (UV Light) Electrophilic Addition (5 types) Oxidation (K 2 Cr 2 O 7 / H+ and distil or reflux (from H 2 SO 4) Polymerization (High Heat, High Temp, Ti. Cl 4 (catalyst) Elimination (Alcohol dehydration) (H+ from H 2 SO 4) C=C Elimination Halogen ( : OH- Base in Alcohol) C=C : Nu Substitution (: OH- (aq) ) OR R-X R-OH

END OF REACTIONS LAST TOPIC ANALYSIS IR MS H-NMR C-NMR

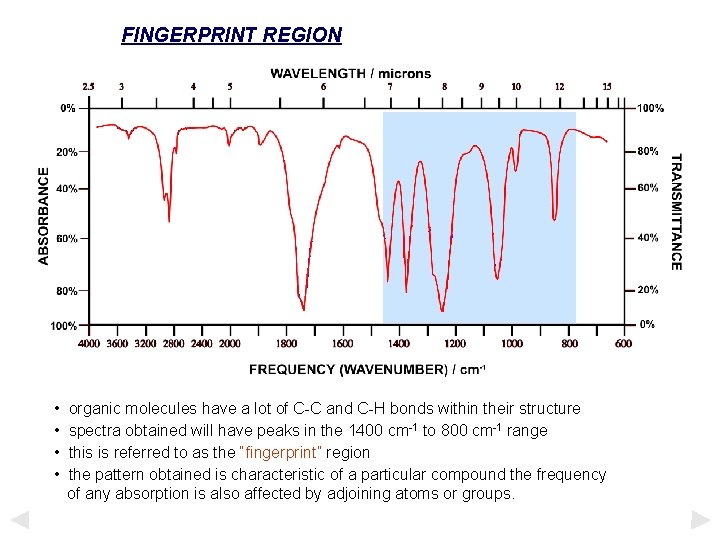
FINGERPRINT REGION • • organic molecules have a lot of C-C and C-H bonds within their structure spectra obtained will have peaks in the 1400 cm-1 to 800 cm-1 range this is referred to as the “fingerprint” region the pattern obtained is characteristic of a particular compound the frequency of any absorption is also affected by adjoining atoms or groups.

7 Mechanisms 1) Elimination (BASE) : OH- (alcohol) Cascade 2) Elimination (Acid) : H+ ( from H 2 SO 4) 3) Free Radical Substitution (UV light) 4) Nucleophilic Substitution : Nu 5) Electrophilic Addition 6) Oxidation: [O] (H+ , K 2 Cr 2 O 7) 7) Polymerization (High temp, high heat, Ti. Cl 4)

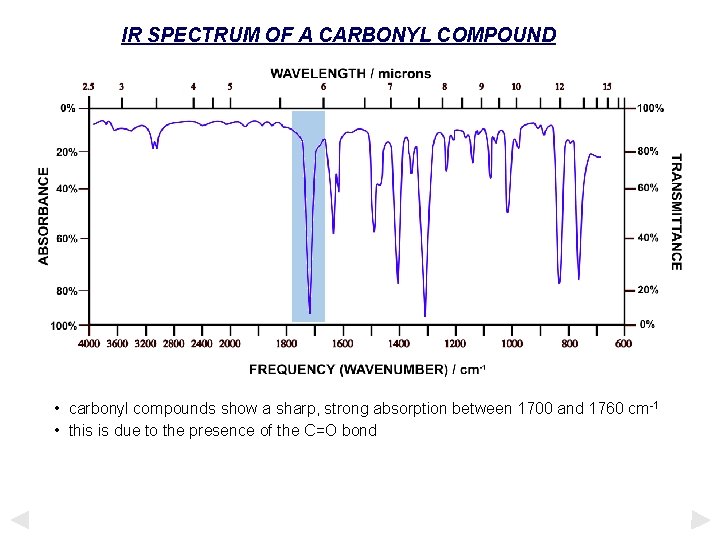
IR SPECTRUM OF A CARBONYL COMPOUND • carbonyl compounds show a sharp, strong absorption between 1700 and 1760 cm-1 • this is due to the presence of the C=O bond

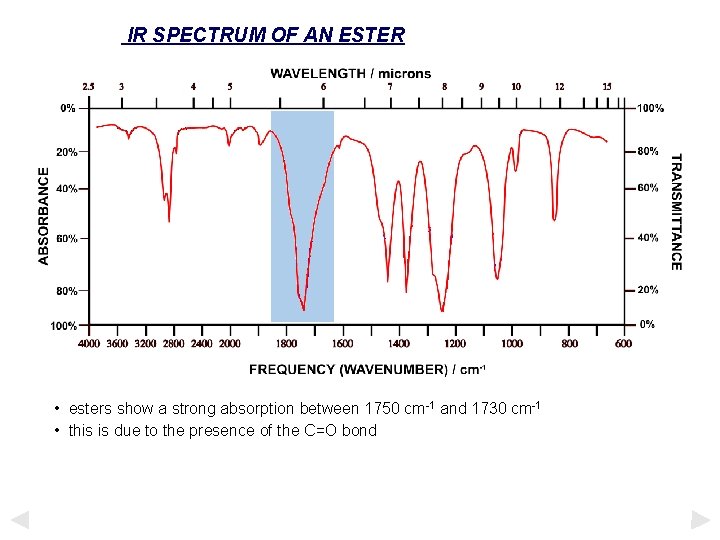
IR SPECTRUM OF AN ESTER • esters show a strong absorption between 1750 cm-1 and 1730 cm-1 • this is due to the presence of the C=O bond

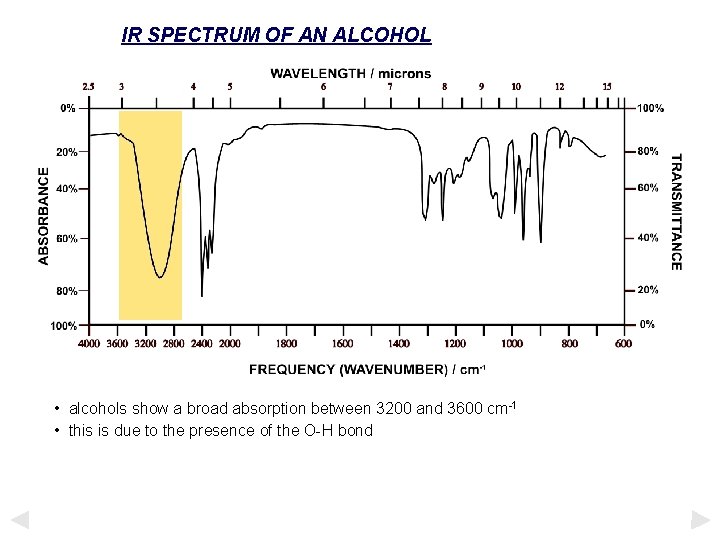
IR SPECTRUM OF AN ALCOHOL • alcohols show a broad absorption between 3200 and 3600 cm-1 • this is due to the presence of the O-H bond

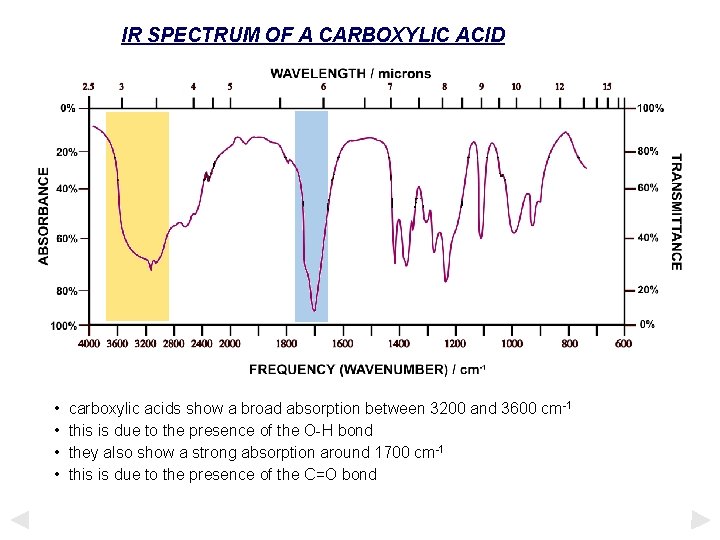
IR SPECTRUM OF A CARBOXYLIC ACID • • carboxylic acids show a broad absorption between 3200 and 3600 cm-1 this is due to the presence of the O-H bond they also show a strong absorption around 1700 cm-1 this is due to the presence of the C=O bond

MASS SPECTROMETRY

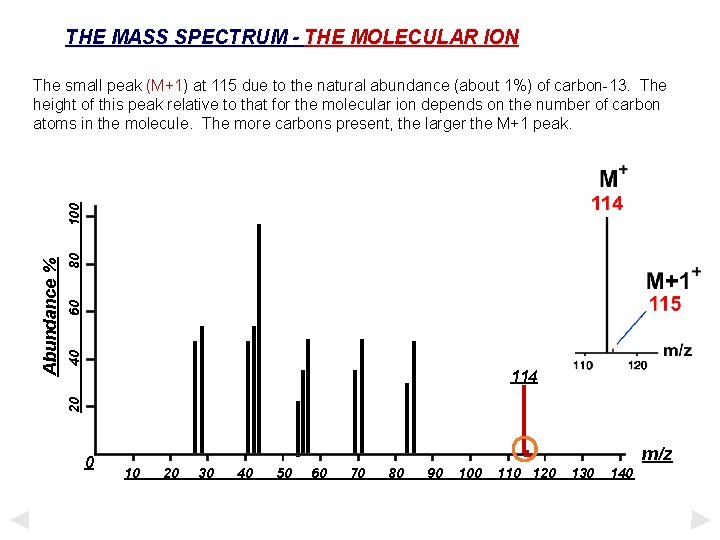
THE MASS SPECTRUM - THE MOLECULAR ION 80 60 40 114 20 Abundance % 100 The small peak (M+1) at 115 due to the natural abundance (about 1%) of carbon-13. The height of this peak relative to that for the molecular ion depends on the number of carbon atoms in the molecule. The more carbons present, the larger the M+1 peak. 0 . 10 20 30 40 50 60 70 80 90 100 110 120 m/z 130 140

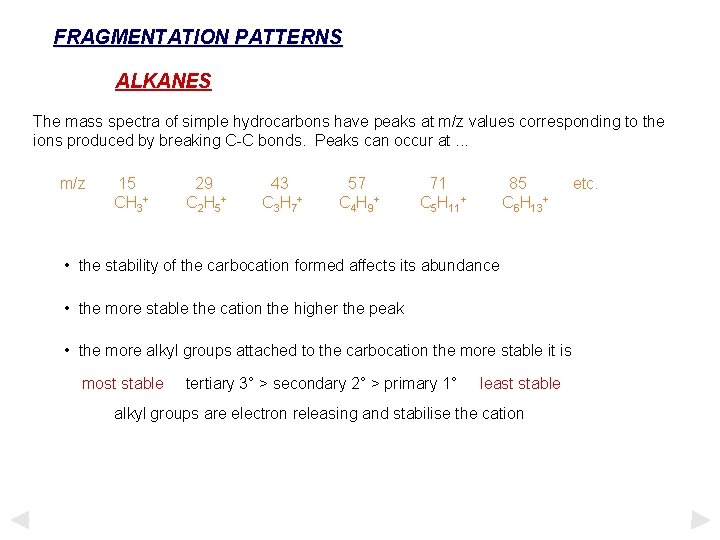
FRAGMENTATION PATTERNS ALKANES The mass spectra of simple hydrocarbons have peaks at m/z values corresponding to the ions produced by breaking C-C bonds. Peaks can occur at. . . m/z 15 CH 3+ 29 C 2 H 5 + 43 C 3 H 7 + 57 C 4 H 9 + 71 C 5 H 11+ 85 C 6 H 13+ • the stability of the carbocation formed affects its abundance • the more stable the cation the higher the peak • the more alkyl groups attached to the carbocation the more stable it is most stable tertiary 3° > secondary 2° > primary 1° least stable alkyl groups are electron releasing and stabilise the cation etc.

Tetramethylsilane Downfield from what? ? ? v. All the protons are chemically equivalent - PRODUCES A SINGLE PEAK v. Non-toxic liquid - SAFE TO USE v. Inert - DOESN’T REACT WITH COMPOUND BEING ANALYSED THE BEST SHEILDING ANYWHERE. WHY? Carbon actually is MORE electronegative than Silicon, thus all the electrons are pulled into the protons (H+) and it is well shielded from external magnetic fields. The molecule contains four methyl groups attached to a silicon atom in a tetrahedral arrangement. All the hydrogen atoms are chemically equivalent. THUS only 1 signal is given off. 56

Chemical Shift (look up on the chart) 1) Ratio of shift downfield from TMS (Hz) Called the delta scale (�� ppm). 2) Based on polarity, the more polar atoms strip away the H+ shielding electrons, leaving them “naked” and frozen in the external magnetic field 3) Unprotected the H+ are held well, thus we need more RF energy to make them resonate. 4) The more polar the more downfield shifted from TMS a molecule is (RCOOH) �� 10 -12 ppm, while CH 3 is 0. 5 ppm 57

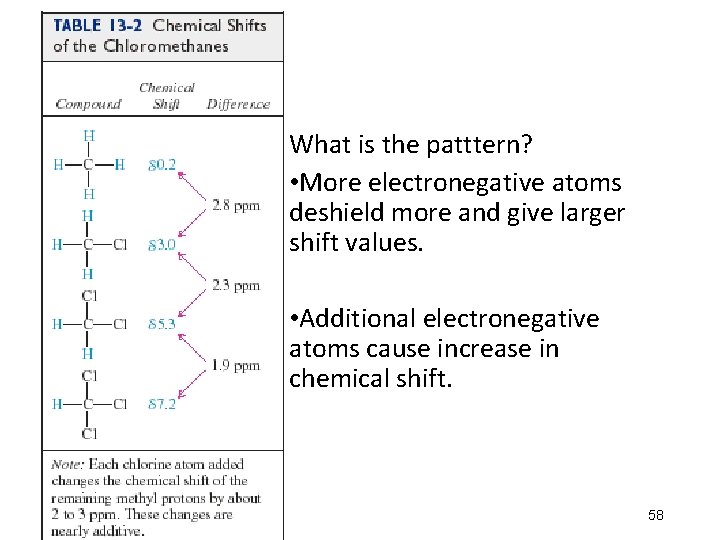
Signals What is the patttern? • More electronegative atoms deshield more and give larger shift values. • Additional electronegative atoms cause increase in chemical shift. 58

Spin-Spin Splitting Protons (H+) on adjacent (neighboring) carbons have magnetic fields that may align with other adjacent protons and this increases their signal. Hydrogen's in similar environments will have the same magnetic spin This magnetic coupling causes the proton peaks to split into doublets, triplets, quintet. (n+1) EXAMPLES of Proton Coupling or peak splitting Singlet has 0 Proton neighbors or n=0 Doublet has 1 Proton neighbor or n=1 Triplet has 2 Proton neighbors or n=2 Quartet has 3 Proton neighbors or n=3 so n+1 = 1 peak so n+1 = 2 peaks so n+1 = 3 peaks so n+1 = 4 peaks 59

n+1 rule Hydrogen's in similar environments will have the same magnetic spin. Ex CH 3 here: CH 3 CH 2 CH 3. The CH 3 will send out the SAME signals they are equivalent. SO, we say we have only 2 environments for hydrogen. The 2 CH 3 have symmetry Different environments in neighboring hydrogen's often do not have the same signal, so they, being different, have slightly different signals. Thus different neighbors interfere slightly with each others signal (called coupling) and cause them to be split (called splitting, note splitting = coupling). They are split in a specific pattern: (splits) = n+1 where n=protons neighbors (non-equivalent) 60

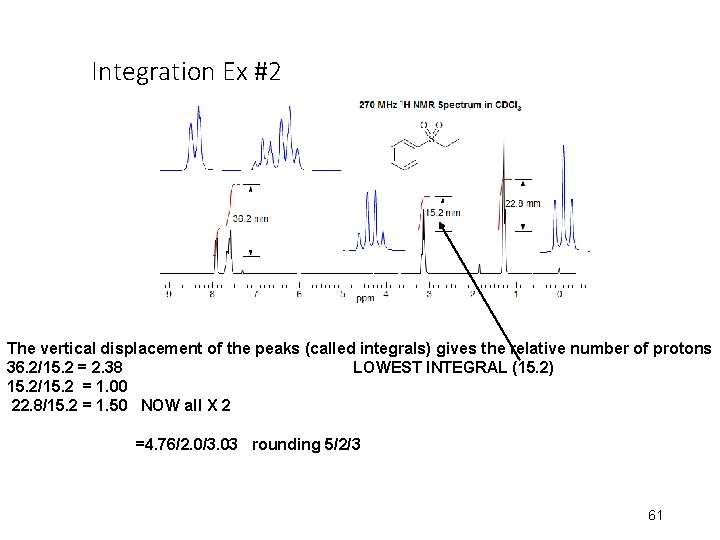
Integration Ex #2 The vertical displacement of the peaks (called integrals) gives the relative number of protons 36. 2/15. 2 = 2. 38 LOWEST INTEGRAL (15. 2) 15. 2/15. 2 = 1. 00 22. 8/15. 2 = 1. 50 NOW all X 2 =4. 76/2. 0/3. 03 rounding 5/2/3 61