PHYSICAL METHODS OF GENE TRANSFERING Transgenic plants What


![[1] ELECTROPORATIONIt is based on Neumann et al (1982) v Electrical impulses of high [1] ELECTROPORATIONIt is based on Neumann et al (1982) v Electrical impulses of high](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-7.jpg)
![[2]LIPOSOME MEDIATED TRANSFORMATION Ø First liposome use for gene transfer was described for delivery [2]LIPOSOME MEDIATED TRANSFORMATION Ø First liposome use for gene transfer was described for delivery](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-8.jpg)
![[3]MICROINJECTION • • • In this process for the genetic conversion, useful genes and [3]MICROINJECTION • • • In this process for the genetic conversion, useful genes and](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-10.jpg)
![[4]SILICON CARBIDE FIBER-MEDIATED TRANSFORMATION • • Silicon carbide fibers (SCF) average 0. 6 um [4]SILICON CARBIDE FIBER-MEDIATED TRANSFORMATION • • Silicon carbide fibers (SCF) average 0. 6 um](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-11.jpg)
![[5] PARTICLE BOMBARDMENT Ø Basic principle – high velocity microprojectile Ø The microprojectile are [5] PARTICLE BOMBARDMENT Ø Basic principle – high velocity microprojectile Ø The microprojectile are](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-12.jpg)

![[6]ULTRASOUND MEDIATED DNA TRANSFORMATION • Ultrasound is used described for stimulating uptake of foreign [6]ULTRASOUND MEDIATED DNA TRANSFORMATION • Ultrasound is used described for stimulating uptake of foreign](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-14.jpg)
![[7]POLYCATION MEDIATED DNA UPTAKE uptake of DNA by protoplast stimulated by – Polyethylene glycol [7]POLYCATION MEDIATED DNA UPTAKE uptake of DNA by protoplast stimulated by – Polyethylene glycol](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-15.jpg)

![[8] ULTRASONICATION This is latest and simple method. -Here plant protoplast, cells in suspension [8] ULTRASONICATION This is latest and simple method. -Here plant protoplast, cells in suspension](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-17.jpg)
![[9] U. V LASER MICROBEAM ØThis is a new method of introducing exogenous gene [9] U. V LASER MICROBEAM ØThis is a new method of introducing exogenous gene](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-18.jpg)
![[10]DNA TRANSFER VIA POLLEN • Pollen has been suggested as a vector for gene [10]DNA TRANSFER VIA POLLEN • Pollen has been suggested as a vector for gene](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-19.jpg)
![[11]Ca-DNA Co-precipitation method • This method was first used for plant transfer in Tobbaco [11]Ca-DNA Co-precipitation method • This method was first used for plant transfer in Tobbaco](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-20.jpg)

- Slides: 22

PHYSICAL METHODS OF GENE TRANSFERING

Transgenic plants What are the transgenic plants? Plants “In which foreign gene or genes enter and that gene integrated with the main DNA of plant, so that the plant Express its phenotype according to the foreign genes” OR “Transgenic plants are produce by introduction of foreign DNA into the plants” EX- BT-cotton, Nicotiana Tobbacum Jaglens regia, Halianthus annus Pisum sativum, Petunia hybrida

In the process of making a transgenic plant, there is a so complicated process have to do, this process have so many techniques and carriers through these we can identify , isolate and can transfer these foreign gene to a desired plant. (1) Identification of a particular gene or gene sequence that Express the specific character. (2) Isolation of gene or gene sequence (3) Use a vector for the transfer of foreign gene OR Vector less transfer of desired genes (physical methods of gene transferring ) Above all steps combined called a process “GENE MANIPULATION”.

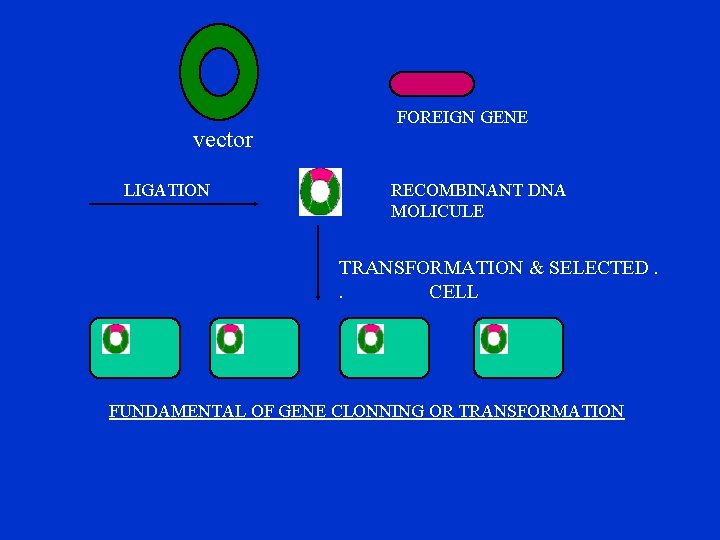
vector LIGATION FOREIGN GENE RECOMBINANT DNA MOLICULE TRANSFORMATION & SELECTED. . CELL FUNDAMENTAL OF GENE CLONNING OR TRANSFORMATION

Physical gene transfer method Why we use this method? Þ Escape to host range limitations can be made by transferring the gene “directly “ without using biological vectors , into the plant cell. Þ Cell wall is an efficient barrier. Þ So Naked cell or protoplast is found best experimental system for study these methods. This is also referred to as DMGT - DNA mediated gene transfer method. The methods are – 1. Electroporation 2. Microprojectile/biolistics/particle bombardment 3. Liposome mediated transformation / Lipofection 4. Microinjection 5. silicon carbide fiber mediated transformation 6. Ultra sound mediated transformation 7. Poly-cation mediated DNA uptake 8. Sonication 9. Ca-DNA Co-precipitation method 10 U. V. laser microbeam 11 Via pollen

Some chemically compound Ex- PEG (poly ethylene glycol), PAV (poly vinyl alcohol), and ca 2 po 4. • In high concentration of solution precipitate the DNA and reverse the permeability of plasma membrane. • In this method protoplast, desired gene and enough chemical material is necessary. Protoplast is the plant cell without cell wall. We can get the protoplast by cellulose enzyme treatment. in this method incubation is conducted with three material protoplast, desired gene, PEG or PVA, during these process foreign genes are uptake by endocytsis. • DESIRED GENES + CHEMICALLY METERIAL +PROTOPLAST TRANSGENIC PLANT
![1 ELECTROPORATIONIt is based on Neumann et al 1982 v Electrical impulses of high [1] ELECTROPORATIONIt is based on Neumann et al (1982) v Electrical impulses of high](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-7.jpg)
[1] ELECTROPORATIONIt is based on Neumann et al (1982) v Electrical impulses of high field strength are used v So that permeable cell membrane can uptake large molecule, Including DNA. v 1 -1. 5 k. V initial field strength is used with a low capacitance and therefore a short decay time. PROCESS- In this procedure a sample of protoplast is pulsed with high /low voltage pulses in the chamber of an electroporation. The chamber is cylindrical in form with a distance of 1 cm Parallel steel electrodes. The pulses is applied by discharge of a capacitor across the cell. Polyethylene glycol (PEG) can increase protoplast transformation frequency. Ø PEG is believed to assist the association of the DNA with membrane. ADVANTAGE - This method is convenient, simple, and fast -Has low cell toxicity. DISADVANTAGE – The disadvantage of the technique is the difficulty in generating plants from protoplast.
![2LIPOSOME MEDIATED TRANSFORMATION Ø First liposome use for gene transfer was described for delivery [2]LIPOSOME MEDIATED TRANSFORMATION Ø First liposome use for gene transfer was described for delivery](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-8.jpg)
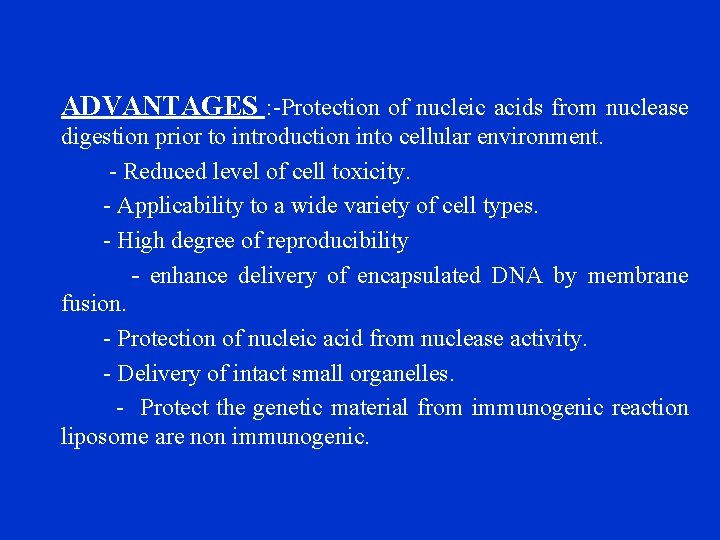
[2]LIPOSOME MEDIATED TRANSFORMATION Ø First liposome use for gene transfer was described for delivery –POLIO VIRUS RNA. Ø The use of liposomes for transformation of transfection system is called lipofection. Ø Liposomes are artificial lipid vesicles surrounded by a synthetic membrane of phospholipids. Ø This membrane have been used in mammalian tissue culture to deliver drugs, proteins, etc. into the cells. Ø These can be induced to fuse with protoplast using PEG and have therefore been used for genetransfer. Ø In this method , DNA enters the protoplasts due to endocytosis of liposome, and this involves the following steps: 1. adhension of liposome to the protoplast surface. 2. fusion of liposome at the site of adhesion. 3. Release of plasmid inside the cell. Ø Liposomes possess many properties akin to those of biological membranes. 1. easy to manipulate 2. lipid contents can be varied 3. many substances may be trapped inside the interlameller spaces. Ex- Lyposome mediated transformation has been demonstrated in protoplast of tobacco and carrot. Liposome mediated transformation showed higher efficiency when it was used in conjunction with PEG. Stability and storage of nucleic acid once encapsulated.

ADVANTAGES : -Protection of nucleic acids from nuclease digestion prior to introduction into cellular environment. - Reduced level of cell toxicity. - Applicability to a wide variety of cell types. - High degree of reproducibility - enhance delivery of encapsulated DNA by membrane fusion. - Protection of nucleic acid from nuclease activity. - Delivery of intact small organelles. - Protect the genetic material from immunogenic reaction liposome are non immunogenic.
![3MICROINJECTION In this process for the genetic conversion useful genes and [3]MICROINJECTION • • • In this process for the genetic conversion, useful genes and](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-10.jpg)
[3]MICROINJECTION • • • In this process for the genetic conversion, useful genes and DNA solution injected Into the protoplast with the help of micropipette (lich ten stein 1987). At the time of injection, protoplast should be immobile. Injected protoplast should be capable for enough culture and new plant origin. In this method normally –embryo, meristematic tissue, pollen grain are uses with this part the chimaeric plants are produce. In chimaeras plants the genetic or DNA composition of organ tissue is different in different organs. further single cell culture technique used to produce plant. by this method transgenic chimera of Brassica napus is produced successfully. • ADVANTAGE – This method frequency of accuracy is very high Approximate 14% than electroporation and Agrobacterium mediated gene transfer.
![4SILICON CARBIDE FIBERMEDIATED TRANSFORMATION Silicon carbide fibers SCF average 0 6 um [4]SILICON CARBIDE FIBER-MEDIATED TRANSFORMATION • • Silicon carbide fibers (SCF) average 0. 6 um](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-11.jpg)
[4]SILICON CARBIDE FIBER-MEDIATED TRANSFORMATION • • Silicon carbide fibers (SCF) average 0. 6 um in diameter and 10 -80 um in length. It has been demonstrated that these fibers have the capability to deliver DNA in to the plant cell. In this method take a mixture in eppendrof tube, mixture contain-silicon carbide fiber explant tissue, plasmid DNA encoding a selectable or screenable marker gene. Silicon carbide fibers have great intrinsic hardness with sharp cutting edges. DNA delivery in this system is presumably due to cell wall penetration by DNA-coated silicon carbide fibers during vortexing of SCF with explant and DNA adhering to fibers might enter the cells. It is possible that SCF function as numerous needles facilitating DNA delivery into the cell. During the mixing process, DNA penetrates the cell to become stably integrated into the nuclear genome. Ex in maize and tobacco this has been demonstrated. • ADVANTAGES : -in this method over other procedures includes • the ability to transform walled cells thus avoiding protoplast isolation, relative ease of the procedure, and very low equipment cost. DISADVANTAGE : - This method has some carcinogenic properties.
![5 PARTICLE BOMBARDMENT Ø Basic principle high velocity microprojectile Ø The microprojectile are [5] PARTICLE BOMBARDMENT Ø Basic principle – high velocity microprojectile Ø The microprojectile are](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-12.jpg)
[5] PARTICLE BOMBARDMENT Ø Basic principle – high velocity microprojectile Ø The microprojectile are commonly DNA coated, gold or tungsten Particles. Ø These accelerated with high initial Velocity (1400 ft /sec) Ø These are normally in 1 -3 um in Diameter. Ø Carried by macroprojectile. Ø Various names like-Particle acceleration method, Bioblaster method, Microprojectile bombardment, Particle gun method, The microprojectile method The gene gun method. Ø The term “BIOLISTIC” has given for this. DIFFERENT BIOLISTIC ACCELERATION MECHANISMGas entertainment - Transferred impulse Macroprojectile acceleration Electrolatic acceleration centripetal acceleration the macroprojectile has proved wide utility.

Several factors might affect the effciency of foreign gene delivery by this. Morphological character of the cells (cell wall thickness, size of cell aggregate) DNA carrying ability of tungsten particle. The effective damage. The degree of expression of the foreign gene in the bombarded cells. ADVANTAGES -: 1. It is tapid and simple procedure. 2. it facilitate the direct transformation of totipotent tissues such as pollen, embryo, meristems and morphogenic cell culture. 3. It appears to be uniquely suitable for organelle transformation. 4. Thousand of particles are accelerated at the same time , causing multiple hits resulting in transfer of genes into many cells simultaneously. DISADVANTAGES -: 1. Requires special equipment. 2. Delivery efficiency still not high. 3. Potential users should be prepared to spend some time adapting existing protocols to their specific species or tissue of interest.
![6ULTRASOUND MEDIATED DNA TRANSFORMATION Ultrasound is used described for stimulating uptake of foreign [6]ULTRASOUND MEDIATED DNA TRANSFORMATION • Ultrasound is used described for stimulating uptake of foreign](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-14.jpg)
[6]ULTRASOUND MEDIATED DNA TRANSFORMATION • Ultrasound is used described for stimulating uptake of foreign DNA by plant protoplasts and leaf segments of tobacco. • In this methods immersion of explant (leaves/protoplasts) in sonication buffer containing plasmid DNA and • Sonication with an ultrasonic pulse generator at an acoustic intensity of 0. 5 w/cm 2 for 30 minute. • Sample are rinsed in a buffer solution. • Then cultured for growth and differentiation. ADVATAGES- This is simple, inexpensive and multifunctional. • One can use standardized condition and there is no requirement for tissue culture expertise. But little success has been achieved by this technique.
![7POLYCATION MEDIATED DNA UPTAKE uptake of DNA by protoplast stimulated by Polyethylene glycol [7]POLYCATION MEDIATED DNA UPTAKE uptake of DNA by protoplast stimulated by – Polyethylene glycol](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-15.jpg)
[7]POLYCATION MEDIATED DNA UPTAKE uptake of DNA by protoplast stimulated by – Polyethylene glycol (PEG) Poly L-lysine Poly L-ornithene or DEAE-dextron with or without metal ions such as Zn, Li, Cs, K, Ca, Na, or Mg etc. ÞPEG is hydrophilic molecule and it removes the “free water ” Þ Free water mean such molecules, which interact with charged molecule soluble in water. ÞThus under high concentration ionic molecule as DNA can no longer stay in solution and get precipitated. Þ DNA as well as protoplast membrane negatively charged, therefore, charge repulsion is obvious. This limits the interaction between DNA and protoplast. Þ At very high concentration , PEG seems to minimize charged repulsion effects and DNA uptake by endocytosis without gross damage to protoplasts. Þ Studies conducted with Petunia , Nicotiana, Rice, Maize etc.

ÞDNA transformation by using the polycation “polybrene” (hexamidethrine bromide) is new, similar techniques are using for transformation of animal cells. ÞIn these experiment PEG was used in combination with pure Ti plasmid or calcium phosphate precipitated Ti plasmid mix with a carrier DNA. ÞMajor advantages of using polybrene technique are the following: - It is less toxic than other techniques - The high transformation efficiency allows very small quantities of plasmid DNA to be used, thus it is easy to introduce only one sequence of genome Since no carrier DNA is used to integrate plasmid DNA into the host genome thus the sequence surrounding the site of integration can be analysed. (!) The transformation frequency appears to be independent of nature of plasmid. (2). The polybrene method can be used for both stable and transient expression.
![8 ULTRASONICATION This is latest and simple method Here plant protoplast cells in suspension [8] ULTRASONICATION This is latest and simple method. -Here plant protoplast, cells in suspension](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-17.jpg)
[8] ULTRASONICATION This is latest and simple method. -Here plant protoplast, cells in suspension and intact pieces of plant tissues can be transformed. -The target cells are suspended in sonication medium in a microcentrifuge tube carrier DNA is added and microtip is immersed in the sonication medium. - Pulse of selected intensity and duration are delivered by homogenizing machine. Transformed cells are finally selected for selection. There are two hypotheses for permeabilization for membrane by this method. 1. First that reversible localized rapture of plamalemma results in is due to violent collapse of activation bubbles which results in generating high-pressure and temprature shock waves to plasmalemma. 2. The second hypothesis predict the existence of critical hydrostatic pressure responsible for reversible mechanical breakdown of membrane. ADVANTAGES: - Equipment for ultrasound transformation is simple, cheap and multifunctional should allow for substantial future applications. -This method has been used in 22% frequency in Tobacco plant.
![9 U V LASER MICROBEAM ØThis is a new method of introducing exogenous gene [9] U. V LASER MICROBEAM ØThis is a new method of introducing exogenous gene](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-18.jpg)
[9] U. V LASER MICROBEAM ØThis is a new method of introducing exogenous gene materials into cells is laser microbeam irradiation. ØDNA incorporated into whole plant cells and applied to Tobacco. ØDNA enters the cell through a self-healing hole made with a laser microbeam. ADVANTAGES: • A laser beam can be used inside the tissues, cells and organelles. • Thus increasing the rate of transformation and the speed of operation. • Wide controllability of the laser beam intensity, flexibility in optical. manipulation. • Wide choice for laser wave length and pulse width are other advantage. • It is also applicable to cells with hard walls. • It can be used for genetic manipulation of plant tissue, individual cells, protoplasts and organelles, • It permits selective fusion in a of part of sub cellular organelle present inside a single living cell.
![10DNA TRANSFER VIA POLLEN Pollen has been suggested as a vector for gene [10]DNA TRANSFER VIA POLLEN • Pollen has been suggested as a vector for gene](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-19.jpg)
[10]DNA TRANSFER VIA POLLEN • Pollen has been suggested as a vector for gene transfer by various workers. • it has been reported that introduction of DNA into gametes followed by fertilization and zygotic embryogenesis will result in gene transfer. • This kind of approach would be simpler faster , and cheaper than the in vitro methods. • Since ovules are difficult to isolate and the injection of DNA into embryo sac in situ seemed to be too tedious and unpredictable, it was logical to favour the use of pollen as a vector for DNA. • The use of DNA-treated pollen as a vector for pollinating fertile plants of maize has been studied. • DNA can be either taken up by germinating pollen and integrated into sperm nuclei or reach the zygote via pollen tube pathway, • The main problems are the presence of cell wall and action of nucleases on the DNA. • Agrobacterium has also been used to transfer the T-DNA via pollen as a vector to overcome the nuclease action on DNA. • Both these approaches have been tried and some success has been achieved.
![11CaDNA Coprecipitation method This method was first used for plant transfer in Tobbaco [11]Ca-DNA Co-precipitation method • This method was first used for plant transfer in Tobbaco](https://slidetodoc.com/presentation_image_h2/bbfe99184a6b4c8a0d4af0ee0b4ada58/image-20.jpg)
[11]Ca-DNA Co-precipitation method • This method was first used for plant transfer in Tobbaco leaf/protoplast by Hein et al (1985). • Basis is the formation of a co-precipitate of plasmid DNA with calcium phosphate, formed by adding cacl 2 to a solution of DNA in phosphate buffer. • On contact with protoplast, co- precipitated is transferred across the plasma memebrane in a calcium requiring process. • In order to encourage the endocytosis uptake of precipitate , the protoplast ca-DNA complex is treated with polyvinyl alcohol and high p. H calcium.

REFRENCES • CHAWLA, H. S, edition second “plant Biotechnology” • JOSHI, P. “Genetic Engineering and Its Applications” • SINGH, B. D. “Biotechnology” • INTERNET
