Physical Equilibrium Relate the concept of equilibrium to
Physical Equilibrium

• Relate the concept of equilibrium to physical and chemical systems. Include: conditions necessary to achieve eqlbm. Additional KEY Terms Closed system Dynamic equilibrium Macroscopic
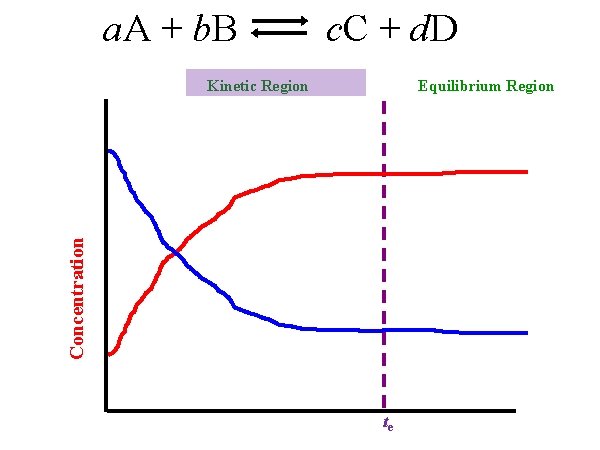
a. A + b. B c. C + d. D Equilibrium Region Concentration Kinetic Region te
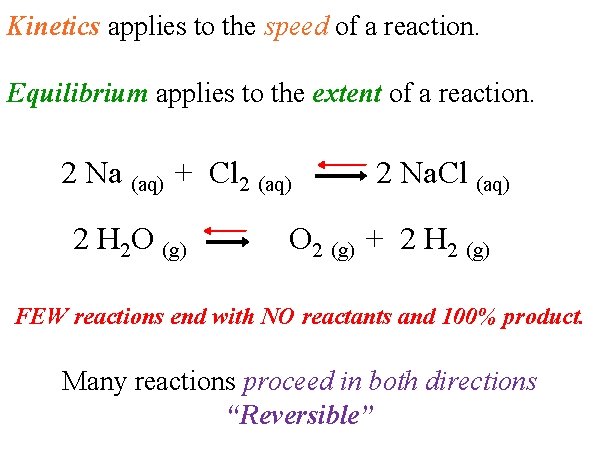
Kinetics applies to the speed of a reaction. Equilibrium applies to the extent of a reaction. 2 Na (aq) + Cl 2 (aq) 2 H 2 O (g) 2 Na. Cl (aq) O 2 (g) + 2 H 2 (g) FEW reactions end with NO reactants and 100% product. Many reactions proceed in both directions “Reversible”
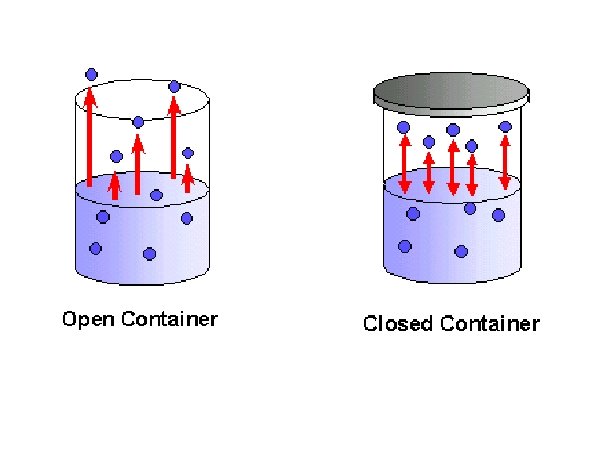
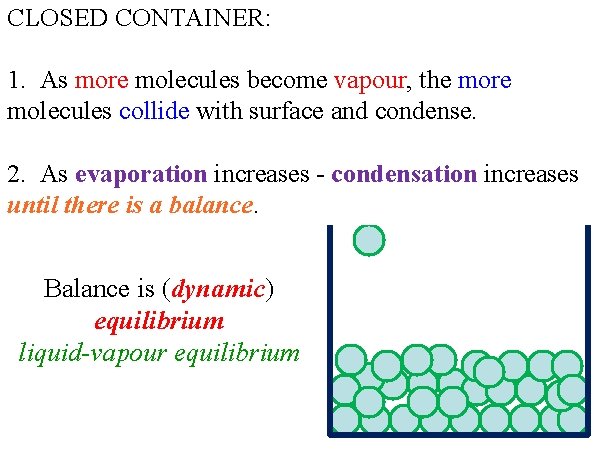
CLOSED CONTAINER: 1. As more molecules become vapour, the more molecules collide with surface and condense. 2. As evaporation increases - condensation increases until there is a balance. Balance is (dynamic) equilibrium liquid-vapour equilibrium
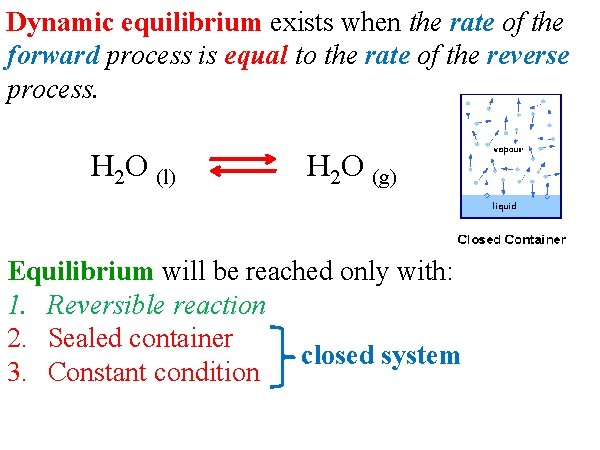
Dynamic equilibrium exists when the rate of the forward process is equal to the rate of the reverse process. H 2 O (l) H 2 O (g) Equilibrium will be reached only with: 1. Reversible reaction 2. Sealed container closed system 3. Constant condition
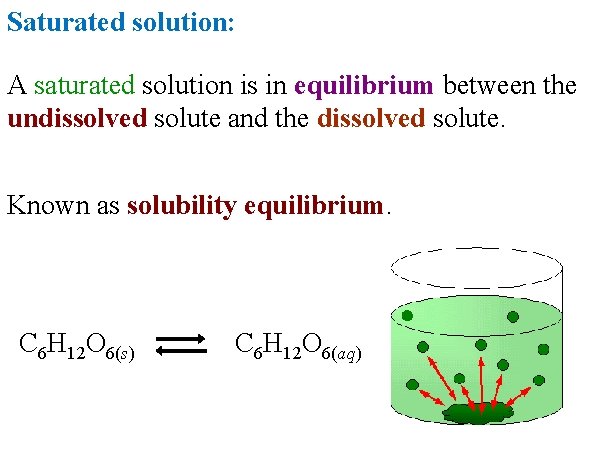
Saturated solution: A saturated solution is in equilibrium between the undissolved solute and the dissolved solute. Known as solubility equilibrium. C 6 H 12 O 6(s) C 6 H 12 O 6(aq)

Any reversible physical process, in which the rate of the forward process is equal to the rate of the reverse process, is known as physical equilibrium. Other examples of physical equilibria include: · Vapour Pressure · Solubility ·Solid-liquid (melting point) · Liquid-vapour (boiling point)

Chemical Equilibrium
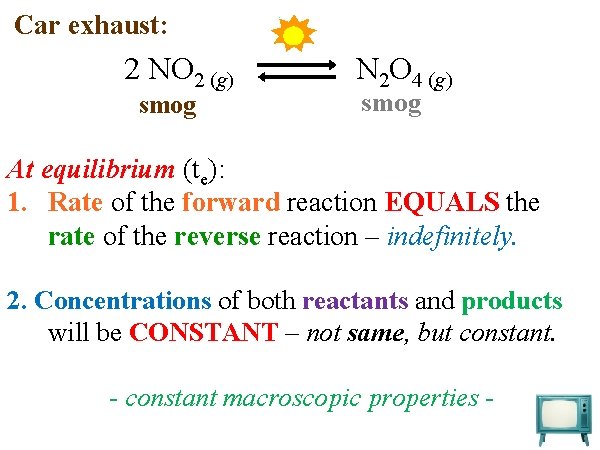
Car exhaust: 2 NO 2 (g) smog N 2 O 4 (g) smog At equilibrium (te): 1. Rate of the forward reaction EQUALS the rate of the reverse reaction – indefinitely. 2. Concentrations of both reactants and products will be CONSTANT – not same, but constant. - constant macroscopic properties -
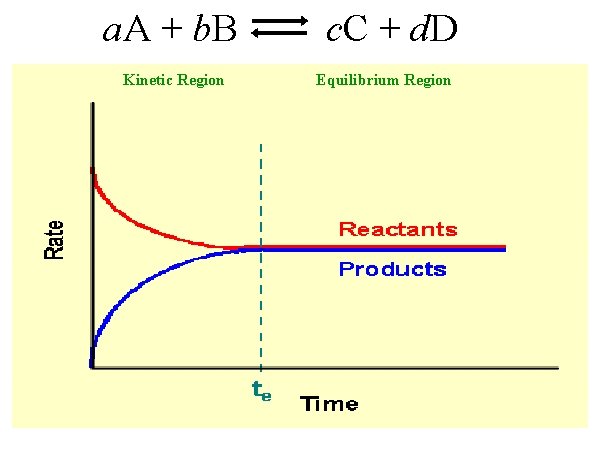
a. A + b. B Kinetic Region c. C + d. D Equilibrium Region
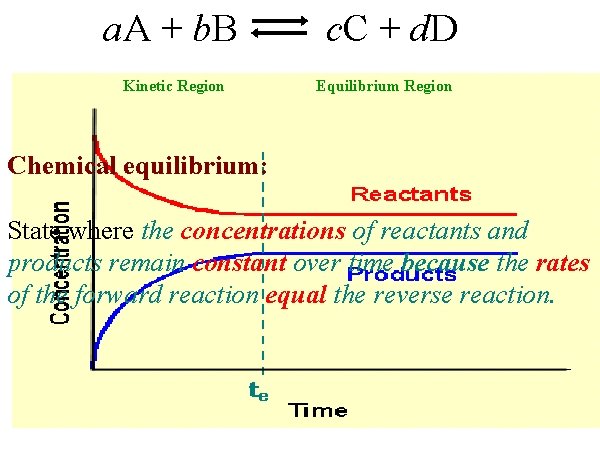
a. A + b. B Kinetic Region c. C + d. D Equilibrium Region Chemical equilibrium: State where the concentrations of reactants and products remain constant over time because the rates of the forward reaction equal the reverse reaction.

CAN YOU / HAVE YOU? • Relate the concept of equilibrium to physical and chemical systems. Include: conditions necessary to achieve eqlbm. Additional KEY Terms Closed system Dynamic equilibrium Macroscopic
- Slides: 14