Physical Chemical Changes Physical Properties Def Properties that





- Slides: 13

Physical & Chemical Changes

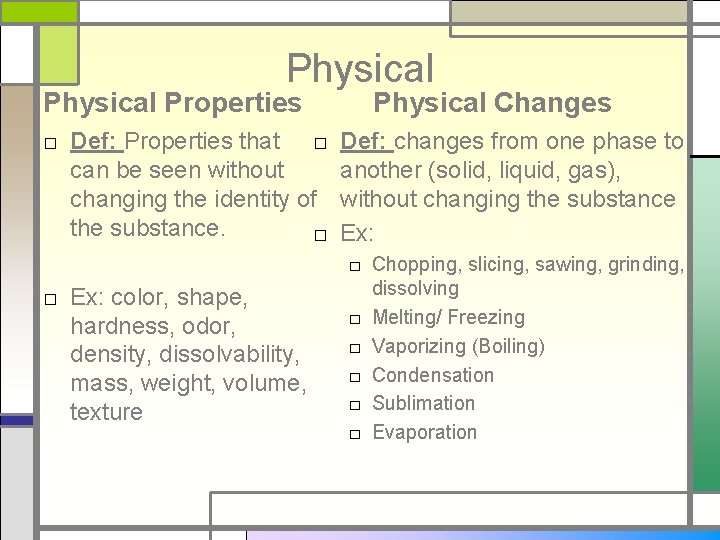
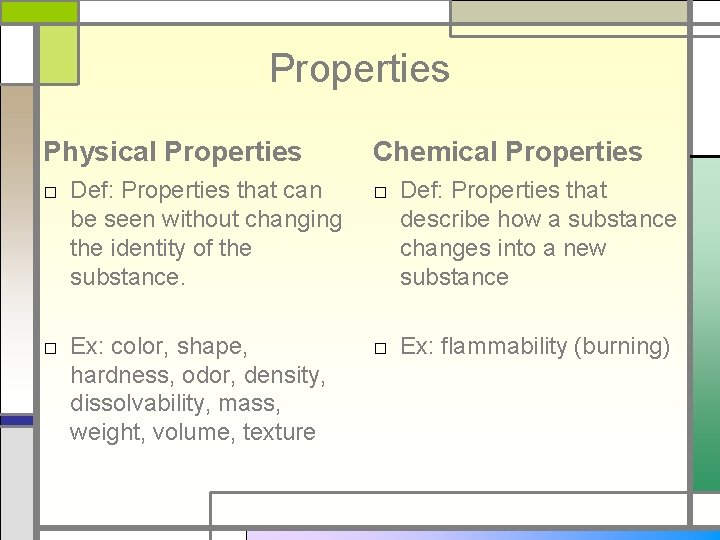
Physical Properties □ Def: Properties that □ can be seen without changing the identity of the substance. □ □ Ex: color, shape, hardness, odor, density, dissolvability, mass, weight, volume, texture Physical Changes Def: changes from one phase to another (solid, liquid, gas), without changing the substance Ex: □ Chopping, slicing, sawing, grinding, dissolving □ Melting/ Freezing □ Vaporizing (Boiling) □ Condensation □ Sublimation □ Evaporation

Physical Change □ A change in the physical property of a substance □ The chemical properties remain the same □ Ex: ice to water, carving wood…

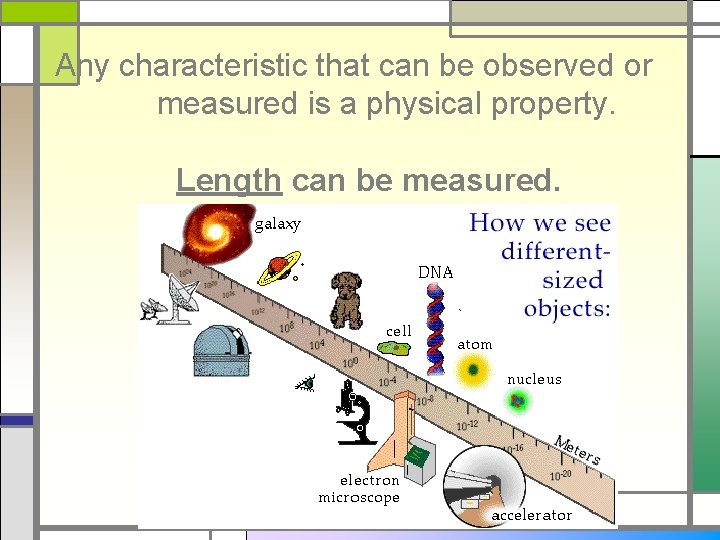
Any characteristic that can be observed or measured is a physical property. Length can be measured.

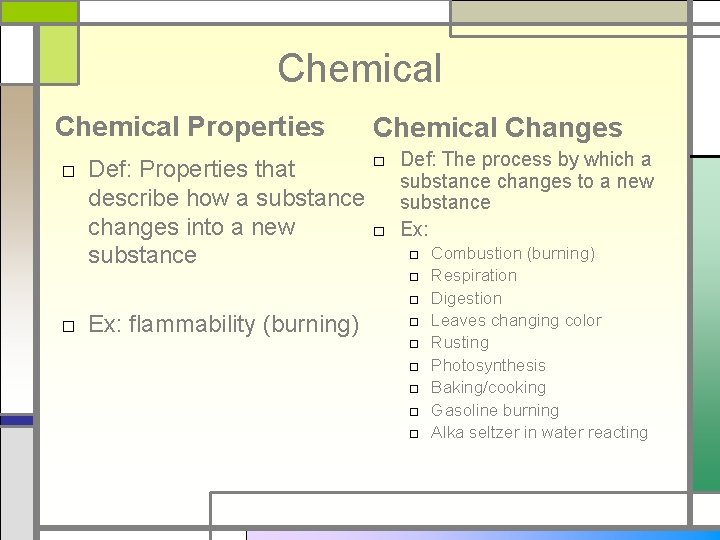
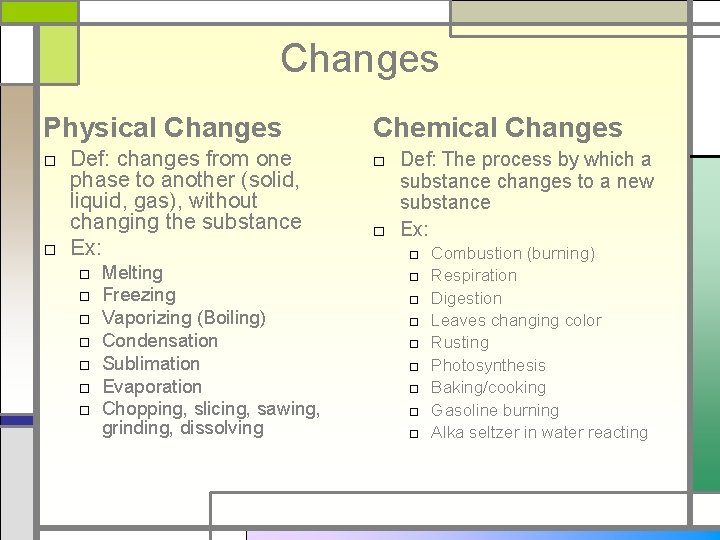
Chemical Properties Chemical Changes □ Def: The process by which a □ Def: Properties that substance changes to a new describe how a substance changes into a new □ Ex: □ Combustion (burning) substance □ Ex: flammability (burning) □ □ □ □ Respiration Digestion Leaves changing color Rusting Photosynthesis Baking/cooking Gasoline burning Alka seltzer in water reacting

Chemical Change □ A substance is transformed into a new substance □ Involves a chemical reaction □ Ex. Iron rusting (with oxygen) to form iron oxide, chemical explosions, burning of wood

Signs of a Chemical Change □ Signs of a chemical change include the production of bubbles, heat, light, smoke, sounds or color changes.

Example Chemical Reaction □ Before During (fire, smoke) □ After change

STUDENT NOTES PAGE

Properties Physical Properties Chemical Properties □ Def: Properties that can be seen without changing the identity of the substance. □ Def: Properties that describe how a substance changes into a new substance □ Ex: color, shape, hardness, odor, density, dissolvability, mass, weight, volume, texture □ Ex: flammability (burning)

Changes Physical Changes Chemical Changes □ Def: changes from one phase to another (solid, liquid, gas), without changing the substance □ Ex: □ Def: The process by which a substance changes to a new substance □ Ex: □ □ □ □ Melting Freezing Vaporizing (Boiling) Condensation Sublimation Evaporation Chopping, slicing, sawing, grinding, dissolving □ □ □ □ □ Combustion (burning) Respiration Digestion Leaves changing color Rusting Photosynthesis Baking/cooking Gasoline burning Alka seltzer in water reacting

DEMOS

Demo #1 Demo #5 Demo #2 Demo #6 Demo #3 Demo #7 Demo #4 Demo #8