PHYSICAL CHEMICAL CHANGES 2 Objectives 1 Compare and

PHYSICAL & CHEMICAL CHANGES 2. Objectives: 1. Compare and contrast physical and chemical changes Identify examples of physical and chemical changes
Why do I care? n Physical and Chemical changes occur around us every day! Understanding them will help us better understand our world.
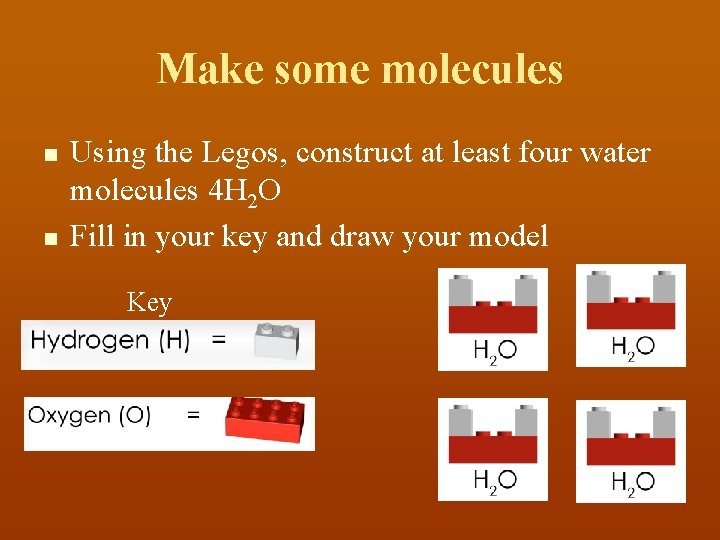
Make some molecules n n Using the Legos, construct at least four water molecules 4 H 2 O Fill in your key and draw your model Key
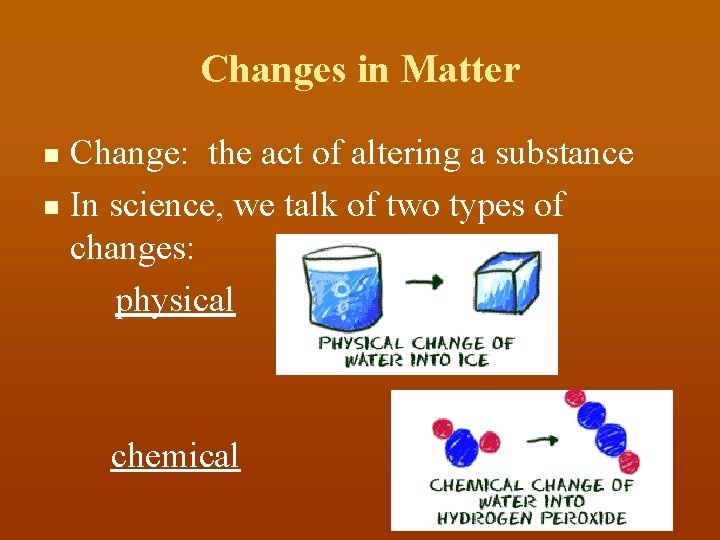
Changes in Matter Change: the act of altering a substance n In science, we talk of two types of changes: physical n chemical
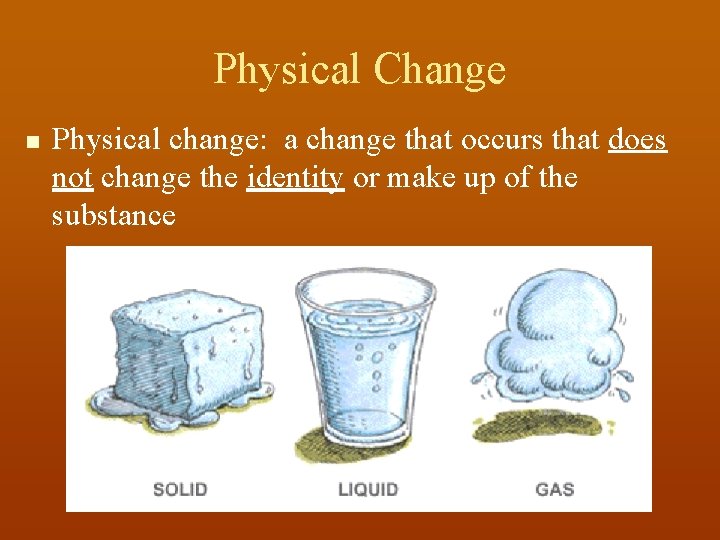
Physical Change n Physical change: a change that occurs that does not change the identity or make up of the substance
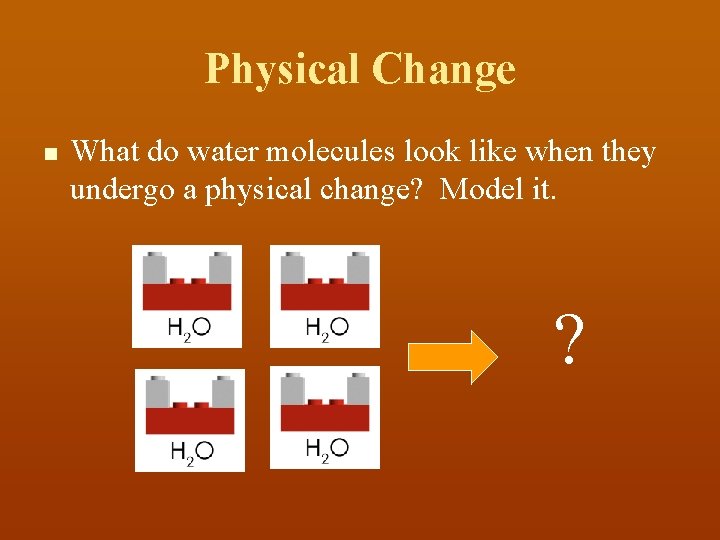
Physical Change n What do water molecules look like when they undergo a physical change? Model it. ?
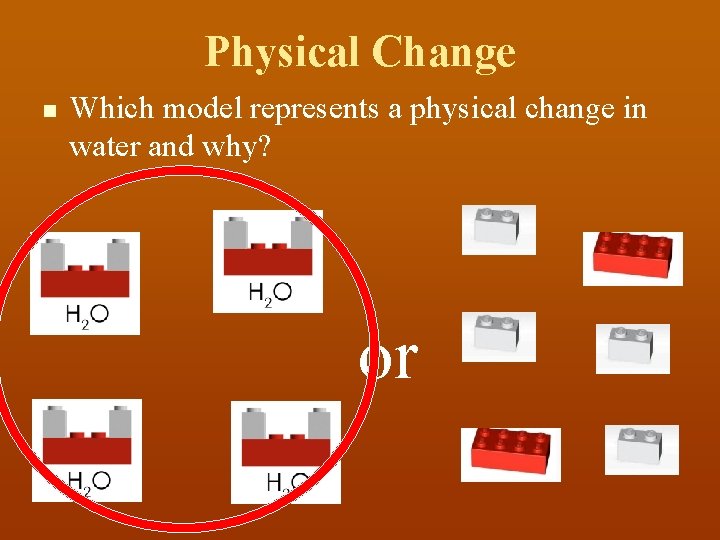
Physical Change n Which model represents a physical change in water and why? or
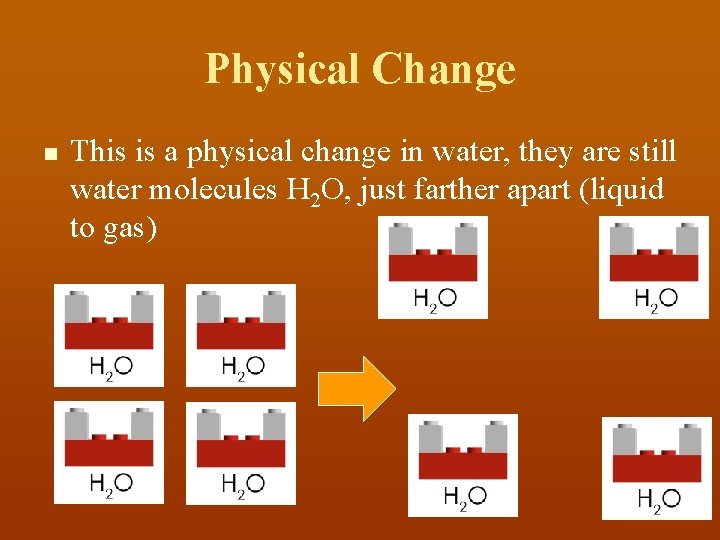
Physical Change n This is a physical change in water, they are still water molecules H 2 O, just farther apart (liquid to gas)

Physical Changes n n Physical changes are changes that alter the size, shape, location or physical state of a substance but not its chemical state. Its still H 2 O- frozen in snowflakes, as liquid in raindrops, or as a gaseous water vapor, it’s chemical formula has not changed.

What Other Kinds of Changes Are Physical? Cutting n Tearing n Folding n Shredding n Shrinking n Enlarging n Changing shape n Change in Phase n Relocating n Rotating n Molding (shaping) n Dissolving n

Chemical Changes n n Chemical change: a change that occurs causing the identity of the substance to change Also called a chemical reaction
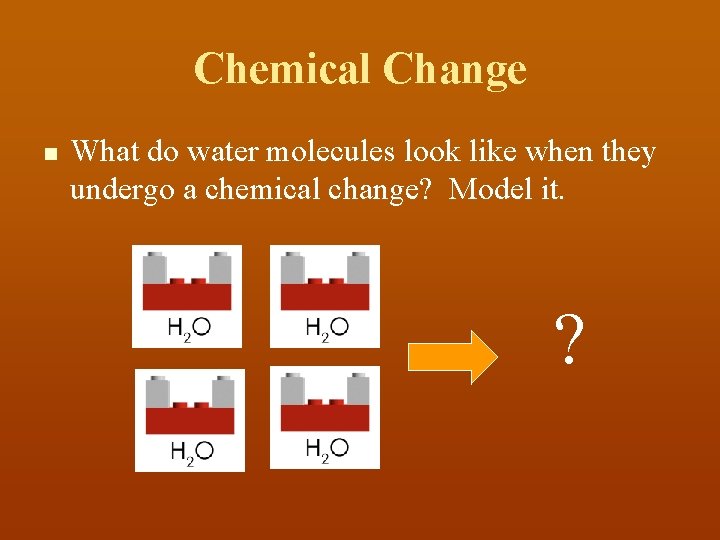
Chemical Change n What do water molecules look like when they undergo a chemical change? Model it. ?
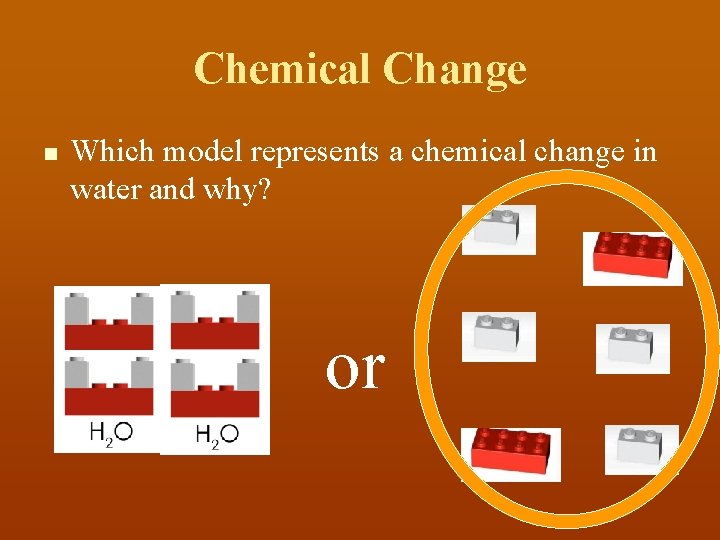
Chemical Change n Which model represents a chemical change in water and why? or
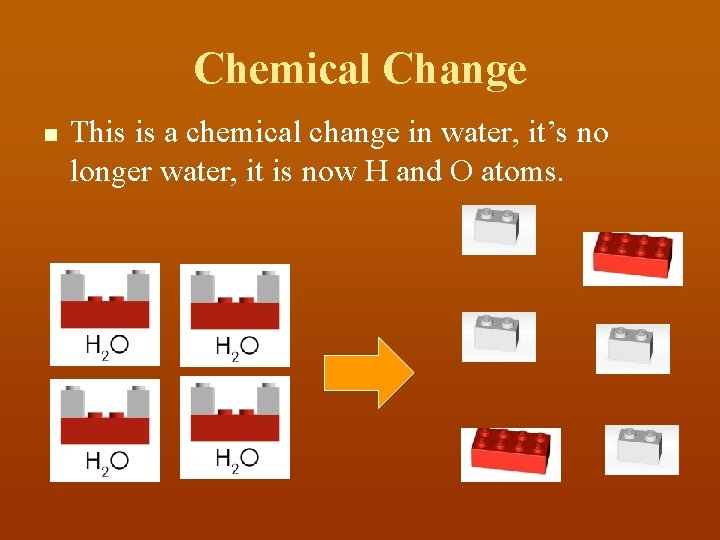
Chemical Change n This is a chemical change in water, it’s no longer water, it is now H and O atoms.
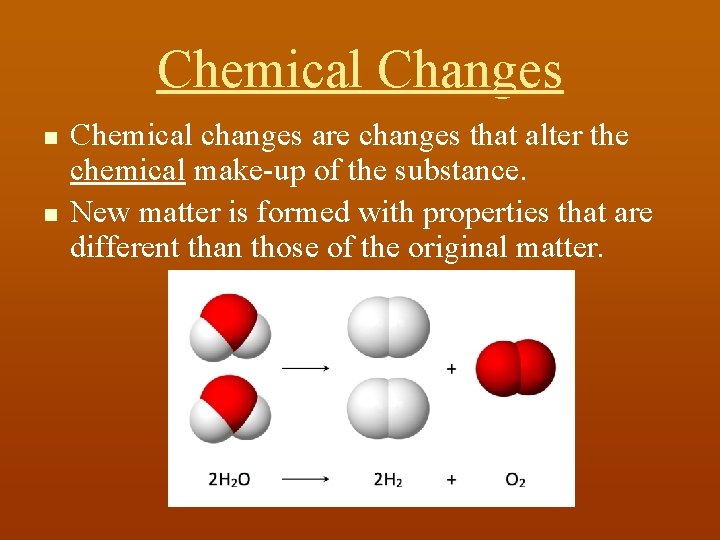
Chemical Changes n n Chemical changes are changes that alter the chemical make-up of the substance. New matter is formed with properties that are different than those of the original matter.

Chemical Changes n If you end up with a chemical or chemicals that you did not start with. . . It is a chemical change!

Examples of chemical changes n n n n burning wood souring milk mixing acid and base digesting food cooking an egg heating sugar to form caramel baking a cake rusting of iron
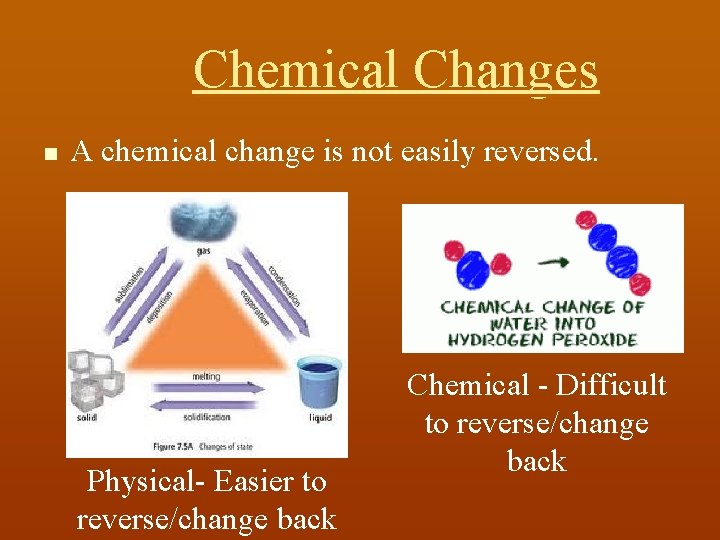
Chemical Changes n A chemical change is not easily reversed. Physical- Easier to reverse/change back Chemical - Difficult to reverse/change back

Signs of a chemical change n n n A color change Production of an odor Change in temperature Formation of bubbles Formation of a solid

Is it Physical or Chemical? Change Melting cheese Burning wood Milk souring Folding up paper Bicycle rusting Physical Chemical
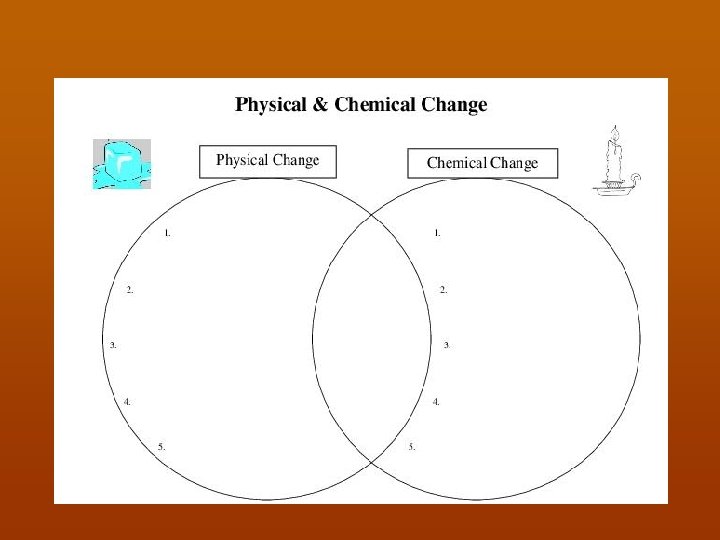

n Start physical changes challenge Powerpoint
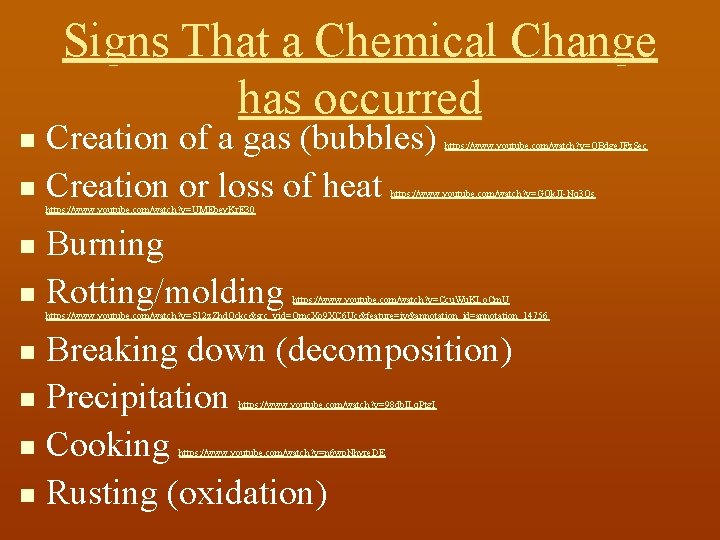
Signs That a Chemical Change has occurred Creation of a gas (bubbles) n Creation or loss of heat n https: //www. youtube. com/watch? v=OBdge. JFz. Sec https: //www. youtube. com/watch? v=GQk. JI-Nq 3 Os https: //www. youtube. com/watch? v=UMEbey. Kr. E 30 Burning n Rotting/molding n https: //www. youtube. com/watch? v=Ccu. Wu. KLo. Cm. U https: //www. youtube. com/watch? v=S 12 z. Zhd. Ockc&src_vid=Omc. Xo 9 XC 6 Uc&feature=iv&annotation_id=annotation_14756 Breaking down (decomposition) n Precipitation n Cooking n Rusting (oxidation) n https: //www. youtube. com/watch? v=98 db. ILq. Ptg. I https: //www. youtube. com/watch? v=n 6 wp. Nhyre. DE

Examples n n A physical change in a substance doesn't change what the substance is. Cutting, folding, or crumpling paper – n A physical change in the shape and size of the paper. However, it is still paper! https: //www. youtube. com/watch? v=k. K 4 EMUd 9 eds n Dissolving sugar in iced tean n Sugar is still C 6 H 12 O 6 but now it is spread out in the liquid, it still tastes just as sweet! https: //www. youtube. com/watch? v=D 1 d. Rd. YPRvp. Y
- Slides: 24