Physical and Chemical Properties Physical and Chemical Changes











- Slides: 13

Physical and Chemical Properties. Physical and Chemical Changes What does the object look like? How does the object change?

What is a property? l Property: a characteristic of a substance that can be observed

Physical Property Physical property: a property that can be observed without changing the identity of the substance. • luster: shininess or dullness • malleability: the ability to be hammered into a thin sheet • ductility: the ability to be stretched into a wire • melting point • boiling point • density

Special Physical Properties l Melting point: the temperature at which a substance changes from a solid to a liquid at a given pressure water = 0 o. C l Boiling point: the temperature at which a substance changes from a liquid to a gas at a given pressure water = 100 o. C

Density l Density is the amount of mass per unit of volume. l Density can be used to identify a substance. l The density of water is 1. 0 g/m. L

Density Calculations l Calculations: D = m/V = g/m. L = g/cm 3 l Ex: A cube has a mass of 2. 8 g and occupies a volume of 3. 6 ml. Would this object float or sink in water? Mass = 2. 8 g Volume = 3. 6 m. L D = 2. 8 g/3. 6 m. L= 0. 78 g/m. L u This object would float in water because its density is less than water (1. 0 g/m. L).

Chemical Properties l Chemical property: a property that can only be observed by changing the identity of the substance Examples: • Combustibility (flammable) • Ability to rust • Reactivity

What is Change? l. Change: the act of altering a substance

Physical Change l Physical change: a change that occurs that does not change the identity of the substance u Melting ice(change in state or phase) u Freezing Kool-aid u Tearing paper u Boiling water (same as melting ice) u Change of State

Chemical Changes l Chemical change: a change that occurs causing the identity of the substance to change u Burning u Digesting food u Reacting with other substances l A chemical change is called a chemical reaction

Chemical Changes Cont’d l Indicators of a chemical change: • Color change • Precipitation • Gas production • Temperature change • Changes in characteristic properties (odor, light given off, etc. )

Precipitation l Precipitation – the solid that forms from a solution during a chemical reaction. l It looks like a cloudy solid in an otherwise clear solution.

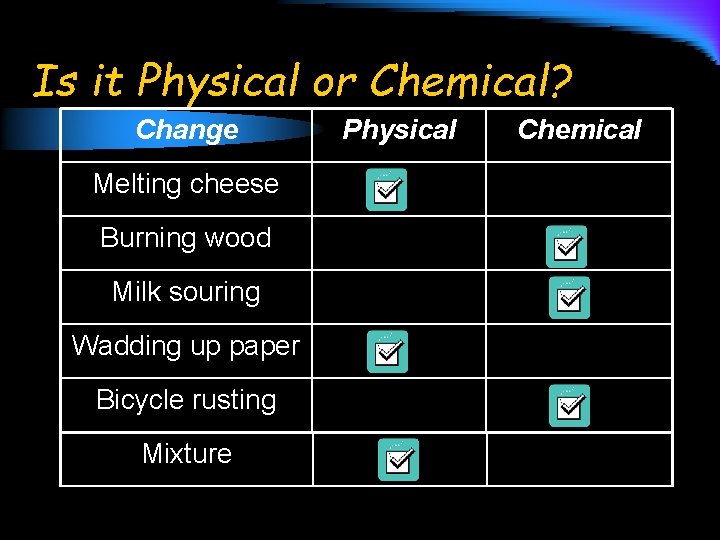
Is it Physical or Chemical? Change Melting cheese Burning wood Milk souring Wadding up paper Bicycle rusting Mixture Physical Chemical