Physical and Chemical Properties of Matter Learning Goals







- Slides: 11

Physical and Chemical Properties of Matter Learning Goals: -I will be able to distinguish the physical and chemical properties of household substances, elements and compounds -I will be able to determine whether a physical or chemical change has occurred

Properties of Matter: Characteristics that help you describe matter are properties. A physical property is one that you can observe without changing that matter into something new e. g. it is a solid at room temp, is colorless, freezes at 0 degrees. A chemical property is one that can be observed when matter is converted into another type of matter e. g. it reacts with oxygen such as the case of rust and iron.

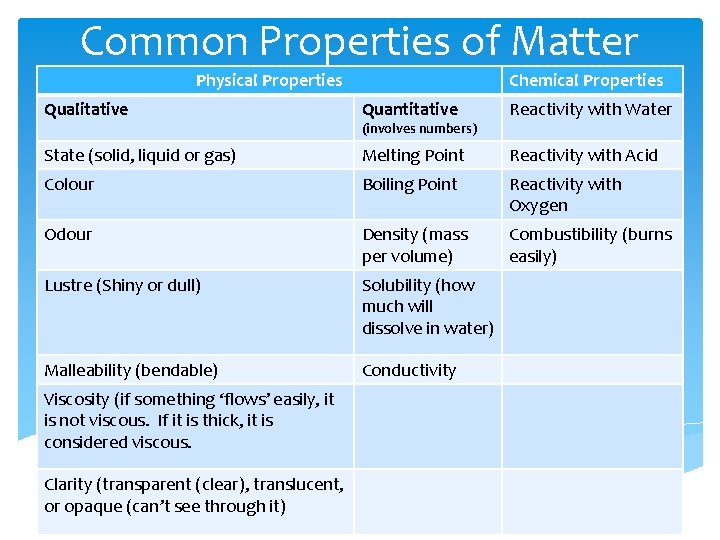
Common Properties of Matter Physical Properties Chemical Properties Qualitative Quantitative Reactivity with Water State (solid, liquid or gas) Melting Point Reactivity with Acid Colour Boiling Point Reactivity with Oxygen Odour Density (mass per volume) Combustibility (burns easily) Lustre (Shiny or dull) Solubility (how much will dissolve in water) Malleability (bendable) Conductivity Viscosity (if something ‘flows’ easily, it is not viscous. If it is thick, it is considered viscous. Clarity (transparent (clear), translucent, or opaque (can’t see through it) (involves numbers)

Characteristics or properties help you describe matter: Properties of Matter http: //video. about. com/chemistry/Physical -and-Chemical-Properties-of-Matter. htm

Calculating Density = Mass/ Volume D = M/V Let’s Try It!

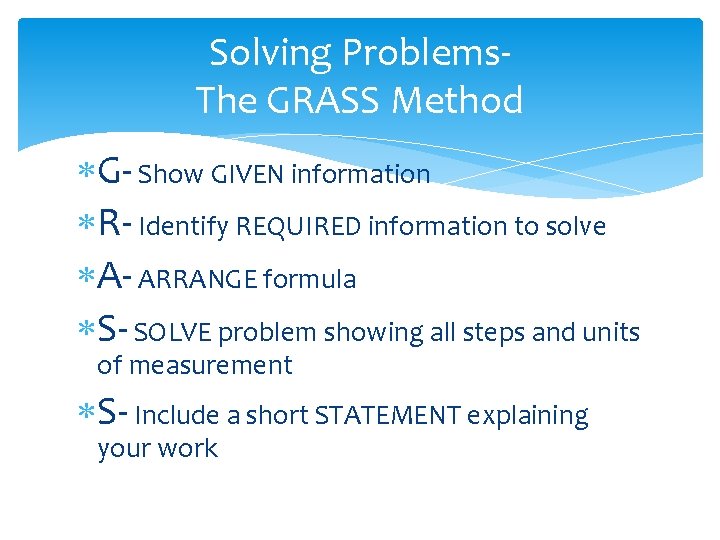
Solving Problems. The GRASS Method G- Show GIVEN information R- Identify REQUIRED information to solve A- ARRANGE formula S- SOLVE problem showing all steps and units of measurement S- Include a short STATEMENT explaining your work

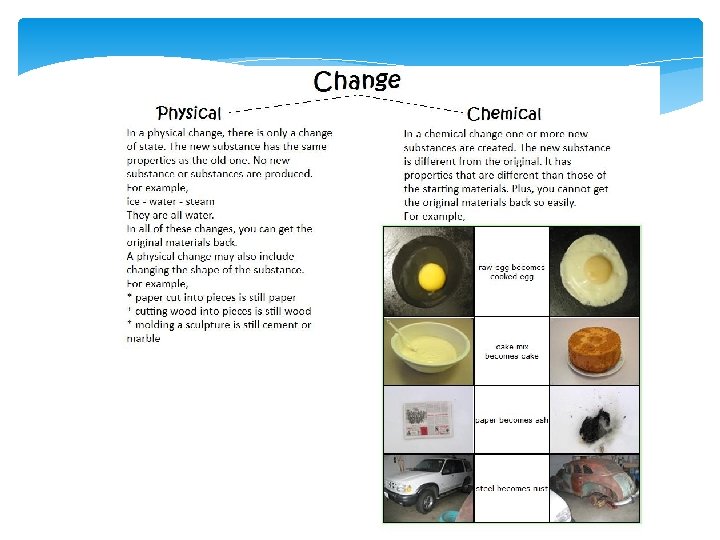
Physical and Chemical Changes Physical Change- Affects the physical appearance of matter but NOT is composition (it remains the same substance. Most physical changes are easily reversible e. g. Ice melting, Kool-Aid dissolving, Cutting paper Chemical Change- Alter the composition of a substance (substance is changed to a new substance) e. g. Burning firewood, baking a cake

5 Clues of Chemical Change A New Colour Appears Bubbles of Gas Being Produced A precipitate is formed (a solid forming from a liquid) Heat/ Light is Produced Difficult to Reverse


Chemical Changes in Action! Can you identify the “Clues of a Chemical Change” in each Reaction? ? Burning Magnesium http: //www. youtube. com/watch? v=8 aqr 1 BO 14 Lw Lead Nitrate and Potassium Iodide http: //www. youtube. com/watch? v=DITY 2 r. XYU-I Sugar and Sulfuric Acid http: //www. youtube. com/watch? v=nq. DHwd 9 r. G 0 s

HOW DID WE DO? Learning Goals: -I will be able to distinguish the physical and chemical properties of household substances, elements and compounds -I will be able to determine whether a physical or chemical change has occurred