Physical and Chemical Properties Lesson 2 Physical Properties












- Slides: 20

Physical and Chemical Properties Lesson 2

Physical Properties n -A physical property describes a characteristic of a substance that can be observed or measured without changing the composition of matter. n Example: Melting Point, Boiling Point

n When water freezes it expands due to a special bonding between water molecules. n n Larger volume with the same mass = Less dense This is why ice floats

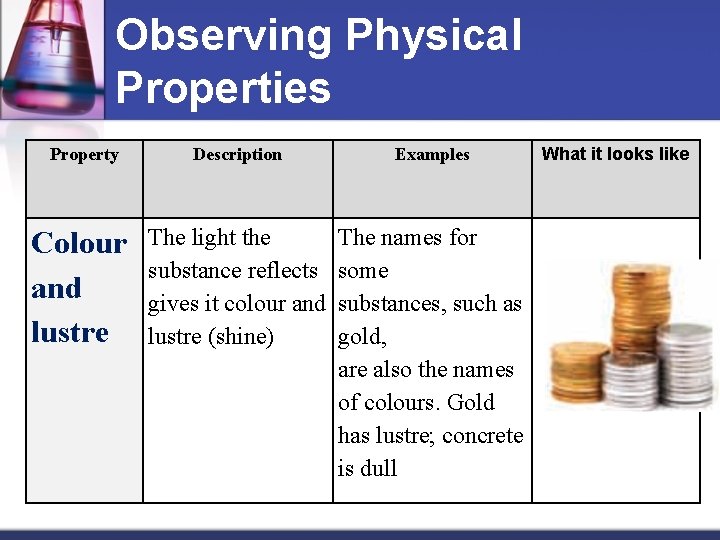
Observing Physical Properties Property Description Examples Colour and lustre The light the substance reflects gives it colour and lustre (shine) The names for some substances, such as gold, are also the names of colours. Gold has lustre; concrete is dull What it looks like

Observing Physical Properties Property Conductivity Description Examples Conductivity is the ability of a substance to conduct electricity or heat. A substance that conducts electricity or heat is called a conductor. A substance with little or no conductivity is an insulator. Most metals are good conductors. Copper is a very good conductor of electricity and so is used to make electric wires. Styrofoam® and glass are insulators. What it looks like

Property Description Density is the Examples The density of amount of pure water is 1 mass in a given g/m. L. volume of The density of a substance. gold is 19 g/m. L. D = m/v Water is denser than oil, but gold is denser than water. What it looks like

Observing Physical Properties Property Ductility Description Examples Any solid that Copper is a can be common stretched into example of a a long wire is ductile said to be material. ductile. What it looks like

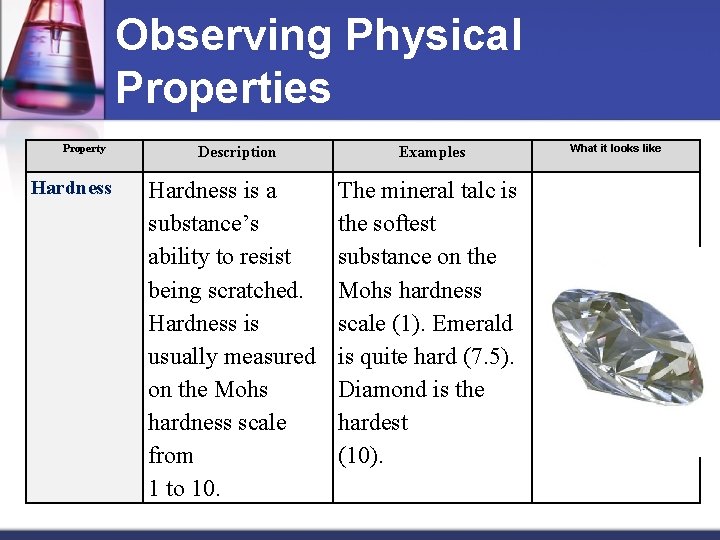
Observing Physical Properties Property Hardness Description Hardness is a substance’s ability to resist being scratched. Hardness is usually measured on the Mohs hardness scale from 1 to 10. Examples The mineral talc is the softest substance on the Mohs hardness scale (1). Emerald is quite hard (7. 5). Diamond is the hardest (10). What it looks like

Observing Physical Properties Property Description Examples Malleability A substance that can be pounded or rolled into sheets is said to be malleable. Aluminum foil is an example of a malleable substance. Metals such as gold and tin are also malleable. What it looks like

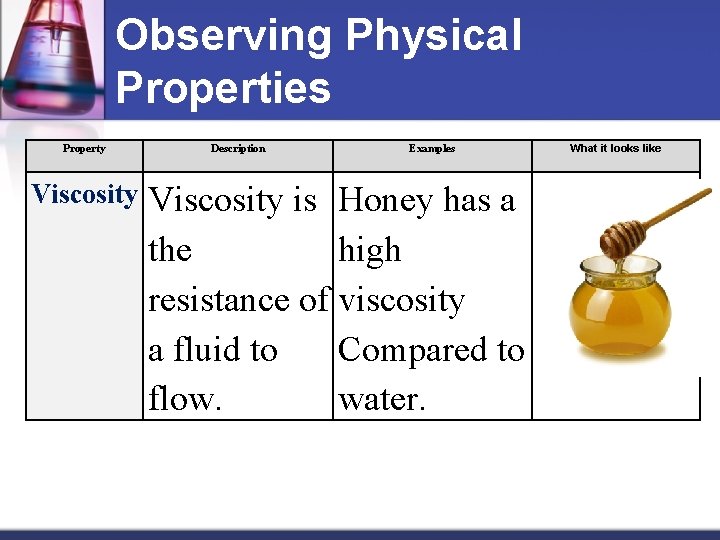
Observing Physical Properties Property Viscosity Description Examples Viscosity is Honey has a the high resistance of viscosity a fluid to Compared to flow. water. What it looks like

Observing Chemical Properties A chemical property describes the ability of a substance to change into a new substance or substances. n In order to view a chemical property a chemical change must occur. n n n Chemical change - the formation of a new substance or substances with new properties. A chemical reaction is a process in which a chemical change occurs.

Chemical Properties 1. Combustibility is the ability of a substance to burn. In order to burn a substance requires Oxygen

Chemical Properties n Light sensitivity is a chemical property of that can cause new substances to form when light hits it.

Chemical Properties 3. Reacting with an acid is a chemical property where when acid is poured on a substance it produces a gas and bubbles.

Physical Change In a physical change, the substance involved remains the same (chemically). The substance may change form or state, however. All changes of state are physical changes. n Examples: Dissolving a substance in a liquid, breaking something. n NOTE: Most physical changes can be reversed. n

Chemical changes In a chemical Change the substance is changed into one or more different substances. n The new substances have different properties from the original substance. n Most chemical changes are difficult to reverse and most cannot be. The new substances are not likely to combine and form the original substance. n

n Often during a chemical change you cannot see the change that has occurred in the substance, but you can observe the results of the change. There are clues that suggest that a chemical change has taken place.

Clues that a chemical change has occurred Clue Evidence Change in colour Final product(s) may have a different colour than the colours of the starting material(s). Formation of a solid (precipitate) Final materials may include a substance in a state that differs from the staring material(s): Precipitate

Clues that a chemical change has occurred Clue Formation of a gas Evidence Final materials may include a substance in a state that differs from the staring material(s); commonly, a gas Release / absorption Energy (light, electricity, sound or most of heat or light commonly heat) is given off or absorbed. The change is difficult The change cannot be reversed or it is to reverse difficult to.

Lab and Practice Questions – hand in