Physical and Chemical Properties and Changes Mrs Storer




























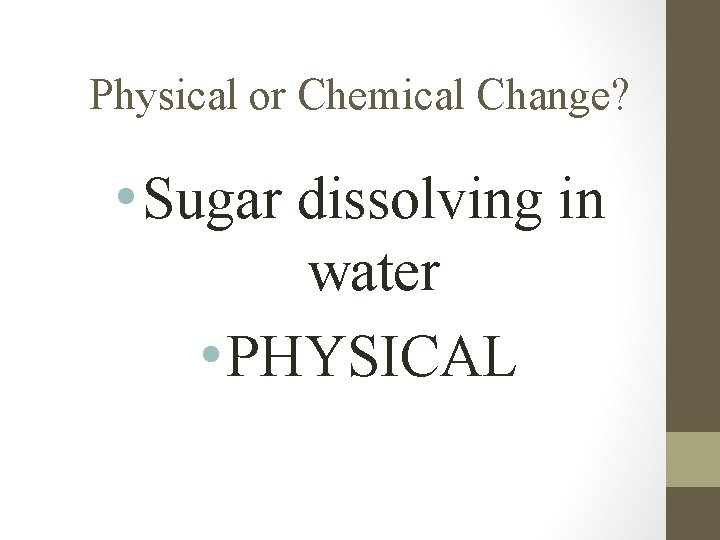







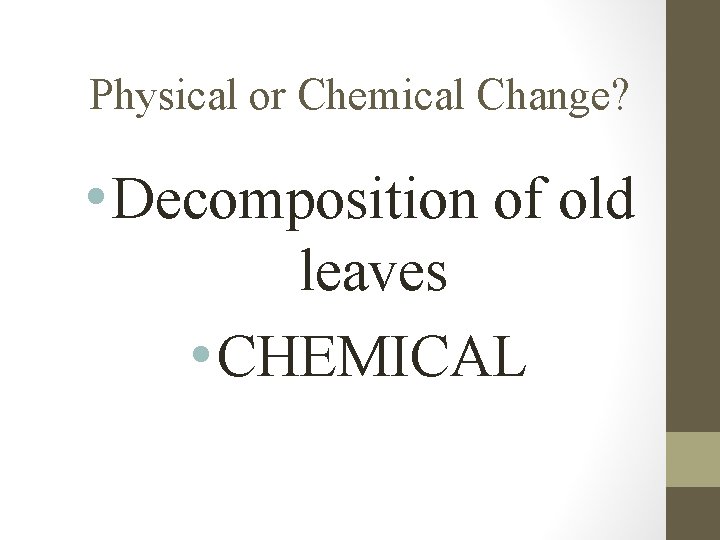


- Slides: 53

Physical and Chemical Properties and Changes Mrs. Storer Chemistry

Physical versus Chemical Properties

MATTER • Matter: anything that has mass and takes up space • Mass – the amount of matter in something • Volume – the amount of space something occupies • Which of the following is matter? • A car? • A box? • You?

What is a property? • Property: a characteristic of a substance that can be observed.

Physical Property Physical property: a property that can be observed without chemically changing the matter. These are usually physical descriptions of the material, but they can also include some behaviors. •

Physical properties can be used to separate mixtures!

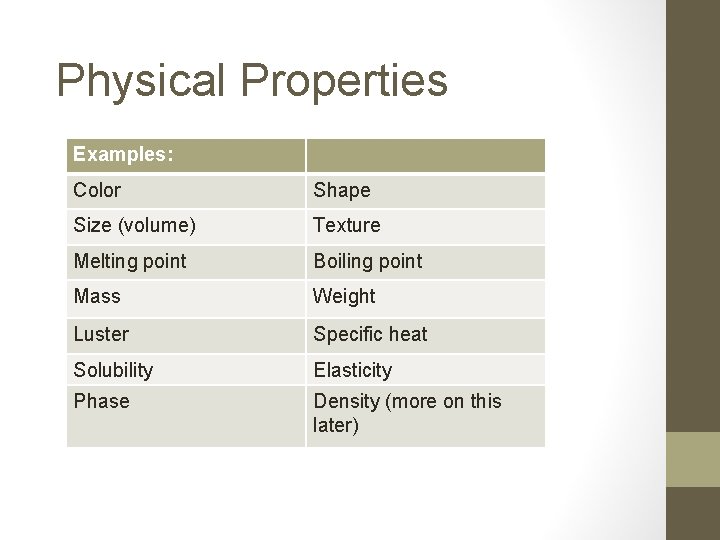
Physical Properties Examples: Color Shape Size (volume) Texture Melting point Boiling point Mass Weight Luster Specific heat Solubility Elasticity Phase Density (more on this later)

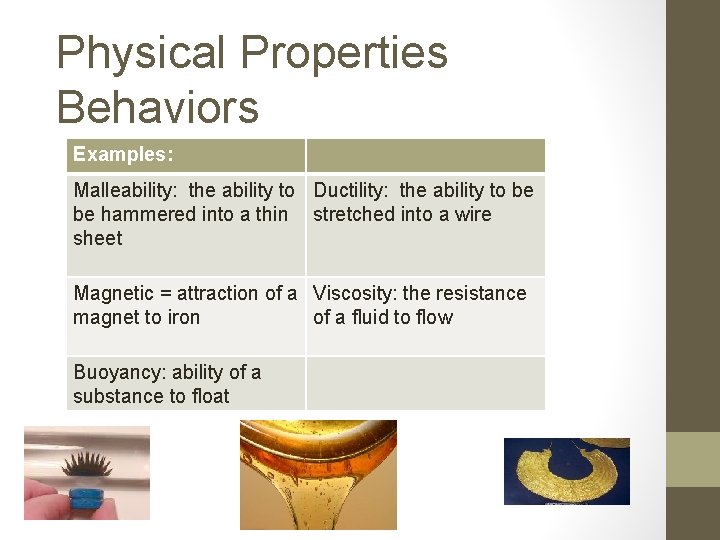
Physical Properties Behaviors Examples: Malleability: the ability to Ductility: the ability to be be hammered into a thin stretched into a wire sheet Magnetic = attraction of a Viscosity: the resistance magnet to iron of a fluid to flow Buoyancy: ability of a substance to float

Extensive vs Intensive Properties Physical properties can be described as: • Extensive – dependent on the amount of the substance • Examples: mass, length, volume • Intensive – independent on the amount • Examples: density, scent Demo: float two pieces of wood of different lengths

Special Physical Properties • Melting point: the temperature at which a substance changes from a solid to a liquid at a given pressure water = 0 o. C • Boiling point: the temperature at which a substance changes from a liquid to a gas at a given pressure water = 100 o. C

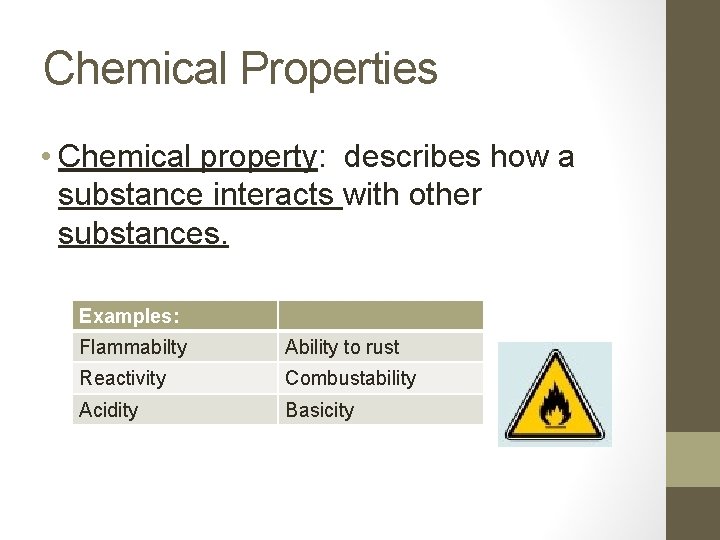
Chemical Properties • Chemical property: describes how a substance interacts with other substances. Examples: Flammabilty Ability to rust Reactivity Combustability Acidity Basicity

FLAMMABILITY: A material’s ability to BURN in the presence of OXYGEN

REACTIVITY: How readily (easily) a substance combines chemically with other substances.

Which has higher reactivity? A 14 karat gold ring or a cheap metal ring from the vending machine at the grocery store? What is your evidence?

Physical and Chemical Change Matter can change in two different ways 1. Physical Change 2. Chemical Change

(1) Physical Change Physical changes are those changes that do not result in the production of a new substance. If you melt a block of ice, you still have H 2 O at the end of the change.

(1) Physical Change Signs of physical change include: • Changing the shape or size • Dissolving • State change

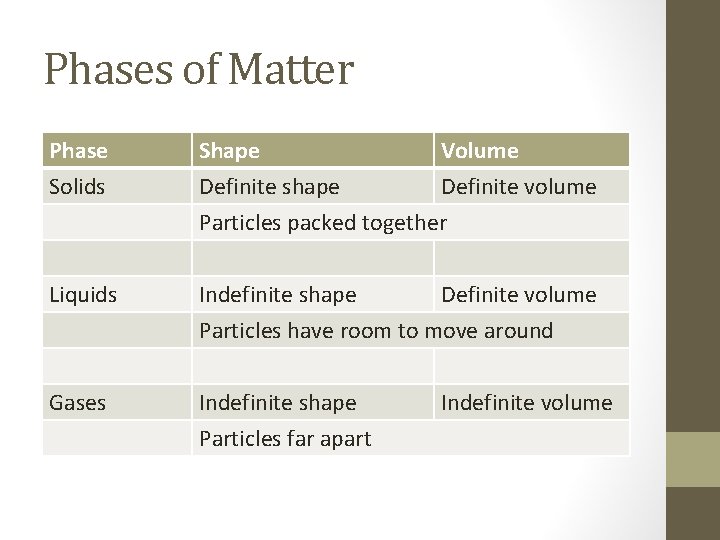
Phases of Matter Phase Solids Shape Volume Definite shape Definite volume Particles packed together Liquids Indefinite shape Definite volume Particles have room to move around Gases Indefinite shape Particles far apart Indefinite volume

Common examples of physical changes are: melting freezing condensing breaking crushing cutting bending

Some, but not all physical changes can be reversed. You could refreeze the water into ice, but you cannot put your hair back together if you don’t like your haircut!

(2) Chemical Change Chemical changes, or chemical reactions, are changes that result in the production of one or more new substances.

Chemical Change Signs of a chemical change include: • gas bubbles • color change - leaves turning colors in the fall, rust appearing • disappearance of color – fading fabric • heat or light

When you burn a log in a fireplace, you are carrying out a chemical reaction that releases carbon. When you light your Bunsen burner in lab, you are carrying out a chemical reaction that produces water and carbon dioxide.

Common examples of chemical changes: digestion photosynthesis decomposition rusting respiration burning tarnishing

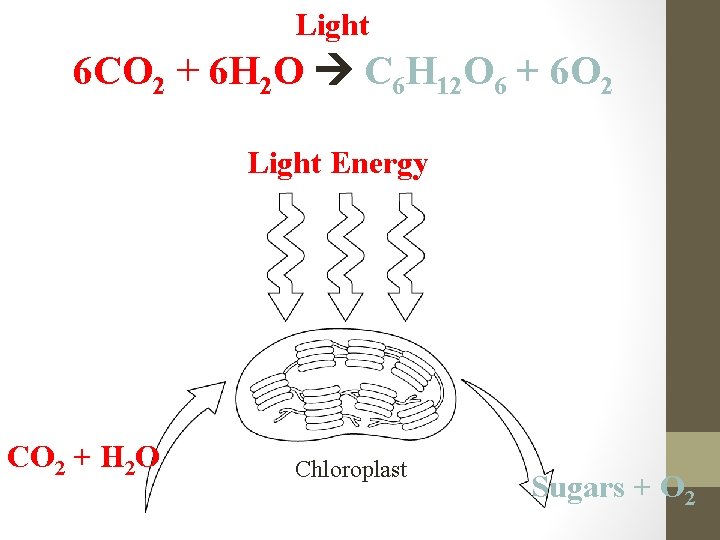
Light 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + 6 O 2 Light Energy CO 2 + H 2 O Chloroplast Sugars + O 2

Chemical Change: Cleaning tarnished silver Tarnish is silver sulfides that form from sulfur compounds in the air

Only sure proof that a new substance is produced is a rapid release of energy – heat, light, and sound

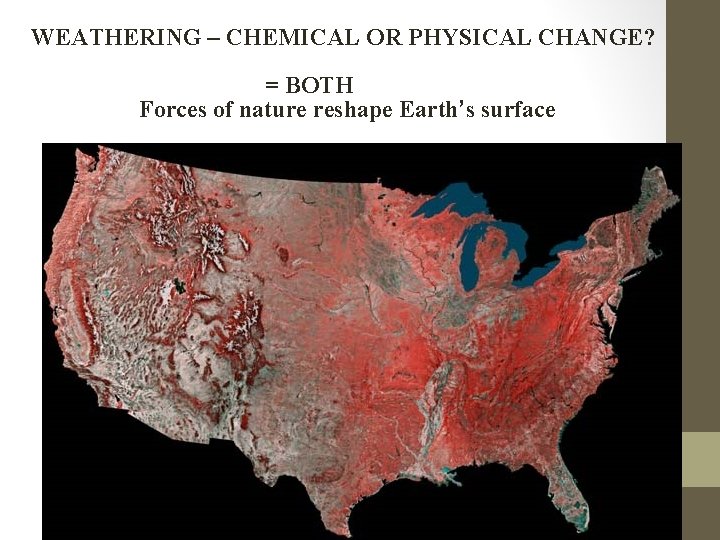
WEATHERING – CHEMICAL OR PHYSICAL CHANGE? = BOTH Forces of nature reshape Earth’s surface

Physical: Large rocks split when water freezes Doesn’t change the rock

Streams cut through softer rock Canyons

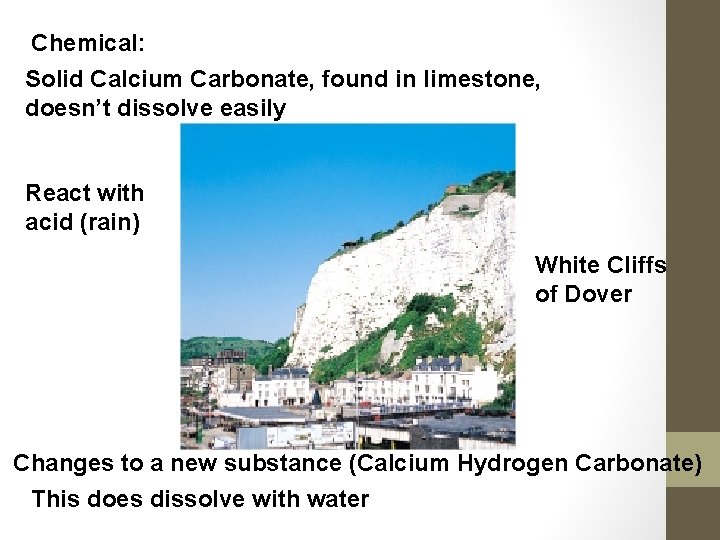
Chemical: Solid Calcium Carbonate, found in limestone, doesn’t dissolve easily React with acid (rain) White Cliffs of Dover Changes to a new substance (Calcium Hydrogen Carbonate) This does dissolve with water

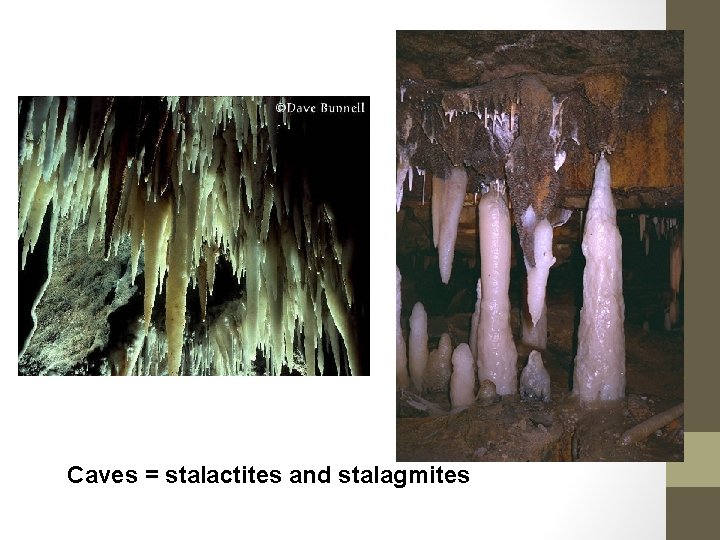
Caves = stalactites and stalagmites

Law of Conservation of Mass Amongst all of this change, remember that Mass cannot be created nor destroyed.

Let’s practice Number your paper from 115 and write if it is a physical or chemical change

Physical or Chemical Change? 1. Painting wood 2. Burning paper 3. Digestion of food 4. Sugar dissolving in water 5. Iron turning red when heated 6. Evaporation 7. Pond freezing in winter

Physical or Chemical Change? 8. Cutting wire 9. Painting fingernails 10. Cutting fabric 11. Baking muffins 12. Shattering glass 13. Decomposition of old leaves 14. Wrinkling of a shirt 15. Old nail rusting

Physical or Chemical Change? • Painting Wood • PHYSICAL

Physical or Chemical Change? • Burning Paper • CHEMICAL

Physical or Chemical Change? • Digestion of food • CHEMICAL
Physical or Chemical Change? • Sugar dissolving in water • PHYSICAL

Physical or Chemical Change? • Iron turning red when heated • PHYSICAL

Physical or Chemical Change? • Evaporation • PHYSICAL

Physical or Chemical Change? • A pond freezing in winter • PHYSICAL

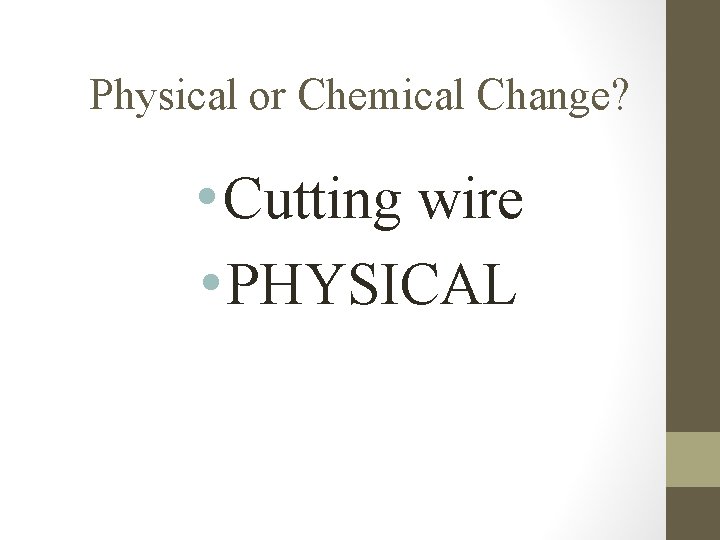
Physical or Chemical Change? • Cutting wire • PHYSICAL

Physical or Chemical Change? • Painting fingernails • PHYSICAL

Physical or Chemical Change? • Cutting fabric • PHYSICAL

Physical or Chemical Change? • Baking muffins • CHEMICAL

Physical or Chemical Change? • Shattering glass • PHYSICAL
Physical or Chemical Change? • Decomposition of old leaves • CHEMICAL

Physical or Chemical Change? • Wrinkling a shirt • PHYSICAL

Physical or Chemical Change? • An old nail rusting • CHEMICAL

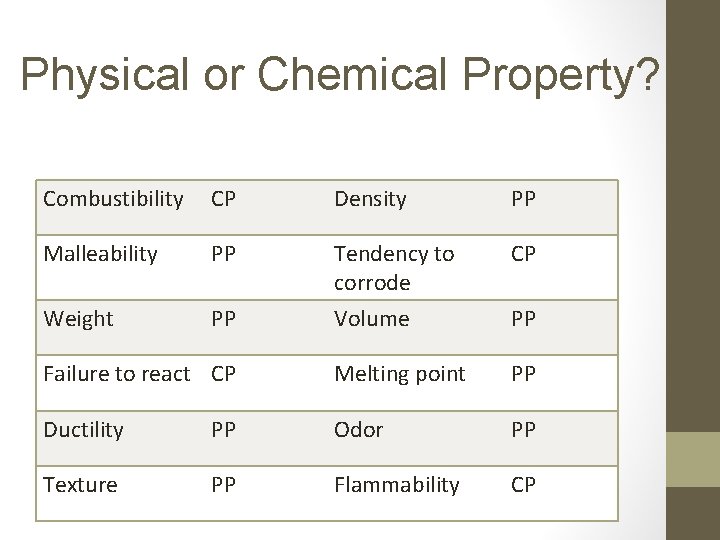
Physical or Chemical Property? Combustibility CP Density PP Malleability PP CP Weight PP Tendency to corrode Volume Failure to react CP Melting point PP Ductility PP Odor PP Texture PP Flammability CP PP

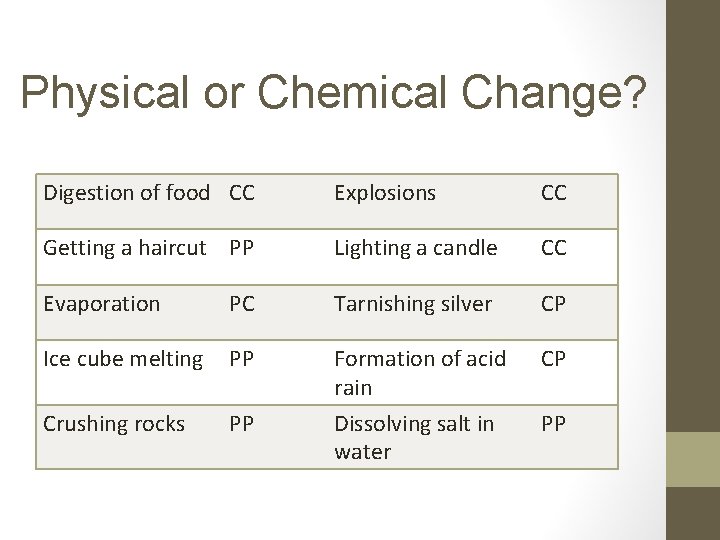
Physical or Chemical Change? Digestion of food CC Explosions CC Getting a haircut PP Lighting a candle CC Evaporation PC Tarnishing silver CP Ice cube melting PP Formation of acid rain CP Crushing rocks PP Dissolving salt in water PP