Physical and Chemical Changes Presented by Kesler Science

Physical and Chemical Changes Presented by Kesler Science Vers. 08/2020 © Kesler Science, LLC

Reflect on the Essential Questions before you dive in… 1. If you were quizzed today, would you already know the answer to the question? Click on and drag a green check mark next to the question. 2. Would you need to learn more about the question to answer confidently? Click on and drag a red “? ” next to the question. Physical & Chemical Changes Essential Questions: 1. How does evidence of chemical reactions indicate that new substances with different properties are formed? © Kesler Science, LLC
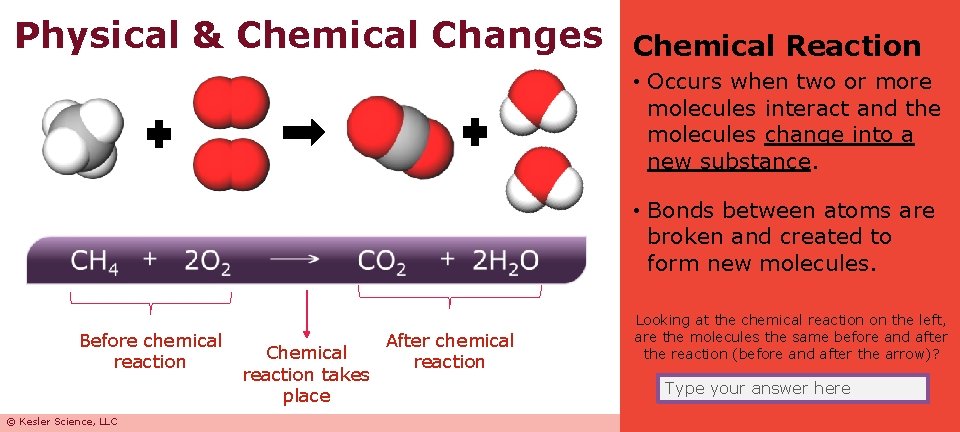
Physical & Chemical Changes Chemical Reaction • Occurs when two or more molecules interact and the molecules change into a new substance. • Bonds between atoms are broken and created to form new molecules. Before chemical reaction © Kesler Science, LLC Chemical reaction takes place After chemical reaction Looking at the chemical reaction on the left, are the molecules the same before and after the reaction (before and after the arrow)? Type your answer here

Physical Change • Changes the form or appearance of a substance Physical & Chemical Changes Name one more example of a physical change. Type your answer here • Does not change substance into anything new • Examples • • Whipping eggs Boiling water Dissolving sugar Dicing vegetables © Kesler Science, LLC
Physical & Chemical Changes What are the physical characteristics of the ice cube? Type your answer here Sm oot h Physical Changes • Changing the state of matter • Changing the color • Changing the temperature • Changing the shape • Changing the texture © Kesler Science, LLC
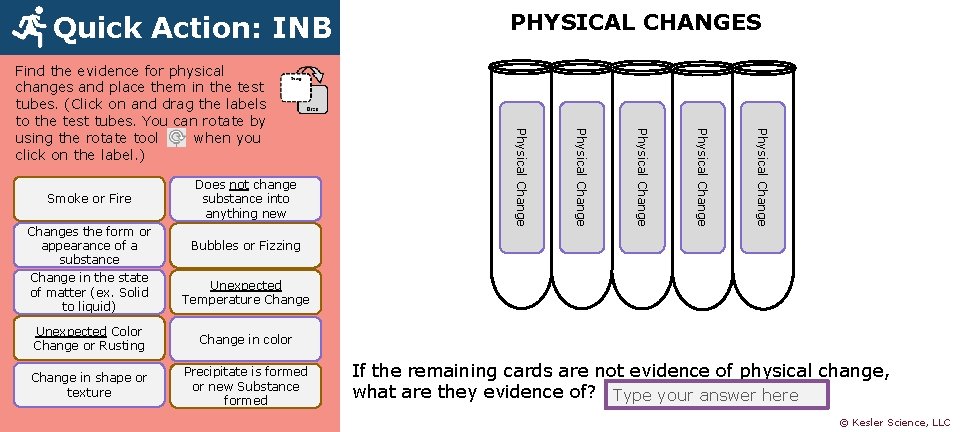
Quick Action: INB Physical Changes the form or appearance of a substance Change in the state of matter (ex. Solid to liquid) Does not change substance into anything new Physical Change Smoke or Fire Physical Change Find the evidence for physical changes and place them in the test tubes. (Click on and drag the labels to the test tubes. You can rotate by using the rotate tool when you click on the label. ) PHYSICAL CHANGES Bubbles or Fizzing Unexpected Temperature Change Unexpected Color Change or Rusting Change in color Change in shape or texture Precipitate is formed or new Substance formed If the remaining cards are not evidence of physical change, what are they evidence of? Type your answer here © Kesler Science, LLC

Chemical Change Physical & Chemical Changes • When two or more substances join to form new substances with new chemical properties • Examples • Iron rusting • Gas burning • Eggs cooking • Bread rising © Kesler Science, LLC
Think About It Type your answer here 1. What is the main difference between a substance going through a physical change and one going through a chemical reaction? 2. Why is making fruit popsicles a physical change? (You can make fruit popsicles by mashing and freezing fruit. ) © Kesler Science, LLC

Physical & Chemical Changes Unexpected Color Change New molecules created in a chemical reaction radiate light differently producing new colors. Examples: • Fireworks • In the fall, leaves change from green to orange and red © Kesler Science, LLC

Examples: • Adding baking soda to vinegar causes the temperature to decrease. • Body temperature increases to fight infections. Physical & Chemical Changes Unexpected Temperature Change During chemical bonding, energy is released or absorbed making their surroundings hotter or cooler In an unexpected temperature change, what causes the temperature change? Type your answer here © Kesler Science, LLC

Physical & Chemical Changes Combustion/Burning A chemical reaction between substances, usually including oxygen accompanied by giving off heat and/or light. This is a permanent change that cannot be undone. Examples: • Wood burning • Candle • Rusting © Kesler Science, LLC

Physical & Chemical Changes Precipitates Form When a solid suddenly appears in a solution and settles to the bottom, collects on another object, or makes the solution cloudy Examples: • Milk souring • Bath tub rings How can you tell that a precipitate has formed? Type your answer here © Kesler Science, LLC

Physical & Chemical Changes Bubbles Form When gases produced in a chemical reaction are released. Examples: • Alka-Seltzer • Bubbles released when vinegar and baking soda are combined © Kesler Science, LLC

Think About It Betty and her grandson decided to bake a cake. When the cake baked, did the ingredients undergo a physical change or chemical reaction? How do you know? Type your answer here They gather eggs, flour, sugar, salt, baking soda, oil, and vanilla. All of the ingredients are measured and mixed following Betty’s grandmother's recipe. They bake the cake in the oven for 30 minutes. © Kesler Science, LLC
Last Look Write a short definition for physical and chemical changes. PHYSICAL CHANGES Type your answer here CHEMICAL CHANGES Type your answer here Then sort the examples of these changes by clicking on and dragging the labels to the correct column. One of each has been done for you. A bicycle sits out in the rain for a year and rusts Melting Ice Mixing 2 substances; a solid is formed at the bottom Combining 2 clear substances; solution created is bright yellow Hammering gold until it becomes flat When boiling water the water turns into steam Tearing Paper Dissolving salt in Change? water Adding an Alka. Seltzer to water and bubbles form Baking a cake in Change? the oven Cracking an egg Change? Burning wood in a Change? campfire Change? Change?

Give your best answer to. . . How does evidence of chemical reactions indicate that new substances with different properties are formed? Type your answer here Check for Understanding Evidence of a chemical reactions: • Unexpected color change • Unexpected temperature change • Combustion/burning • Precipitates form • Bubbles form

Which parts of the essential question do you still need help to understand? Type your answer here Still have questions?
- Slides: 17