Physical and Chemical CHANGES Physical changes are all




























- Slides: 30

Physical and Chemical CHANGES

Physical changes are all about energy and states of matter

• In a physical change there is only a change of state—thermal energy is added or removed. • The new substance has the same chemical properties as the old one. • No new substance(s) are produced. • Physical changes can be reversed.

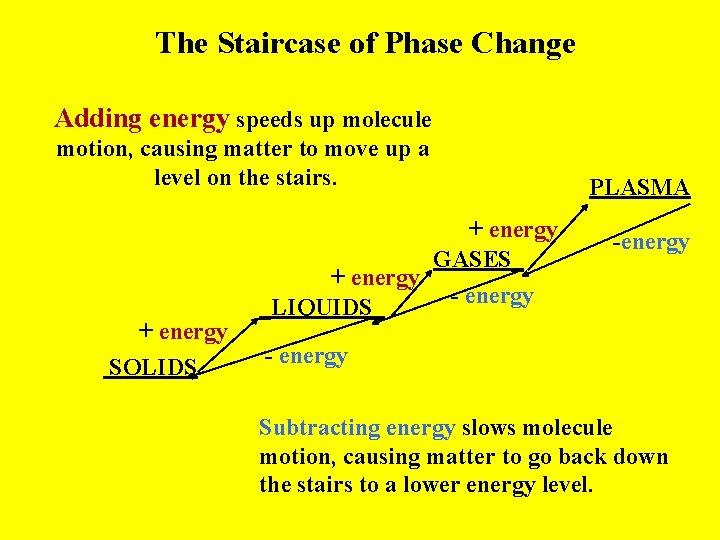
The Staircase of Phase Change Adding energy speeds up molecule motion, causing matter to move up a level on the stairs. + energy SOLIDS + energy GASES_ + energy - energy _LIQUIDS_ PLASMA -energy - energy Subtracting energy slows molecule motion, causing matter to go back down the stairs to a lower energy level.

Chemical changes happen on a molecular level.

• In a chemical change one or more NEW substances are created. • The new substance is different from the original. • It has properties that are different than those of the starting materials. • Plus, you cannot get the original materials back easily.

Identify the following events as: Physical Change Ask yourself: 1. Is the material still the same basic material? 2. Is it just a change of state or phase? 3. Is it really new or different material? 4. Can it be easily reversed? Chemical Change Ask yourself: 1. Are there any new or different substances? 2. Is the material different from the original? 3. Are the chemical properties different? 4. Can it be easily reversed?

squeezing oranges to make orange juice burning toast cooking spaghetti glass breaking burning leaves frying an egg mixing Kool-Aid powder into water melting Popsicles hammering wood together to build a playhouse freezing chocolate covered bananas melting butter for popcorn pouring milk on your cereal separating sand from gravel spoiling food bleaching your hair making salt water to gargle with fireworks exploding cream being whipped

Glow Sticks

Cooking applesauce

Whipping cream

Adding milk to cereal

Burning leaves

Making frozen chocolate bananas

Hammering wood together

Bleaching your hair

Dissolving Kool-Aid in water

Spoiled food

Melting a popsicle

Breaking a glass

Exploding fireworks Fourth of July Ashland, KY

Melting butter for popcorn

Cooking an egg

Separating sand gravel

Burned toast

Squeezing oranges for juice

Quiz Time!

Use these to complete the questions: Chemical Energy New Reversed 1. In a physical change there is only a change of state—_______ is added or removed. 2. The new substance has the same _____ properties as the old one. 3. No _____ substance(s) are produced. 4. Physical changes are easily ____.

Use these to complete the statements: Cannot Different New Original 1. In a chemical change one or more _____ substances are created. 2. The new substance is different from the _____ substance. 3. It has properties that are _______ than those of the starting materials. 4. You _______get the original materials back easily.

The END