PHYS 221 FINAL EXAM REVIEW SESSION Good news











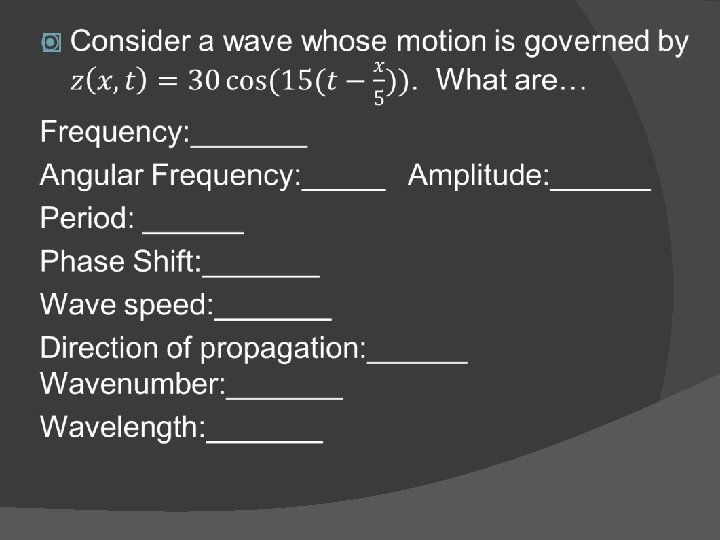
![Traveling wave Y(x, t) = (1. 00 cm)cos[(10. 00 rad/m)x + (30. 0 rad/s)t]. Traveling wave Y(x, t) = (1. 00 cm)cos[(10. 00 rad/m)x + (30. 0 rad/s)t].](https://slidetodoc.com/presentation_image/06c3c23fc958b12fa8ee5aa663c706d5/image-12.jpg)






















- Slides: 41

PHYS 221 FINAL EXAM REVIEW SESSION


Good news, bad news… � I’ll be the Phys 222 SI Leader

How to participate in this review session � Don’t just sit there. � When I solve problems I’ll try to write up the generic equation that I’m using first, then plug in numbers. Generic equations will be in blue. All other work is in some other color. � When you see how I’m solving the problem, try to look up the equation on the equation sheet.

DON’T JUST SIT THERE

Exam Overview “Approximately 1/3 of the problems will stress understanding of the physics concepts, whereas the remainder will be numerical problems to test ability to apply these concepts. ” -Syllabus 27 Questions

Verbatim from blackboard � 11 -12 questions on the material covered in exams 1 and 2 � 11 -12 questions on the material that has not been tested yet (i. e. , after exam 2) � 4 questions about the laboratories.

Lectures covered since exam 2 • • • • Simple Harmonic Motion Pendulum, damped and forced oscillations Sound waves. Energy, intensity. Resonance Interference. Standing waves. Beats. Doppler Temperature. Thermometers. Thermal expansion Heat and phase changes Heat transfer Equations of state. Ideal gas. Kinetic model Phase diagrams First law of thermodynamics Thermal processes. Heat capacities Heat engines and refrigerators Second law of thermodynamics. Carnot cycle Entropy

Breakdown…

Estimates of what’s most important (from me) � Doppler effect � Entropy � Thermal expansion � Thermal equilibrium � Waves along a string � First law � Simple Harmonic Motion

SHM � A block of mass 6 kg is attached to a spring, and the block is stretched out 30 cm before being released. If the period of the oscillation is 10 seconds, what is the spring constant?
![Traveling wave Yx t 1 00 cmcos10 00 radmx 30 0 radst Traveling wave Y(x, t) = (1. 00 cm)cos[(10. 00 rad/m)x + (30. 0 rad/s)t].](https://slidetodoc.com/presentation_image/06c3c23fc958b12fa8ee5aa663c706d5/image-12.jpg)
Traveling wave Y(x, t) = (1. 00 cm)cos[(10. 00 rad/m)x + (30. 0 rad/s)t]. �What is the velocity of the wave? (remember magnitude and direction) �What is the upward velocity of the point x=2 on the string at t=3?

� The molar mass of nitrogen is 14 g/mol. Assume that it can be modeled as a monatomic ideal gas. If you have 2 kg of nitrogen at 30 degrees Celsius, �What is the total energy of the gas? �What is the average velocity of the gas particles? �What is the specific heat of the gas? �What is the root-mean-square velocity of the gas particles?

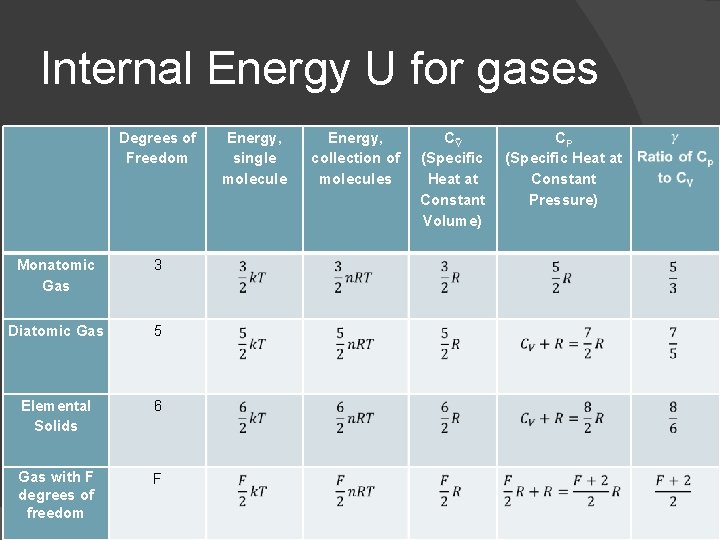
Internal Energy U for gases Degrees of Freedom Monatomic Gas 3 Diatomic Gas 5 Elemental Solids 6 Gas with F degrees of freedom F Energy, single molecule Energy, collection of molecules C V (Specific Heat at Constant Volume) CP (Specific Heat at Constant Pressure)

Examples… �

Doppler Effect � You’re running away from your crying sister (producing a sound at 3000 Hz) at a speed of 5 m/s. She’s running towards you at a rate of 3 m/s. What frequency do you hear?

Sound waves � A continuous succession of sinusoidal wave pulses are produced at one end of a very long string and travel along the length of the string. The wave has frequency 10 Hz seconds, amplitude. 2 m , and the string has mass 12 kg and a length of 500 meters and the tension through the string is 800 N. � Write an equation describing the wave: � How long does it take the wave train to travel 5 m along the string? � How long does it take a point on the wave to travel a distance of 5 m along the string?


SHM � A block of mass 5 kg is attached to a spring, and the block is stretched out 40 cm before being released. �If the period of the oscillation is 5 seconds, what is the spring constant? � Write an equation describing the spring if at t=0 the block is at x=40 cm.

Beats �

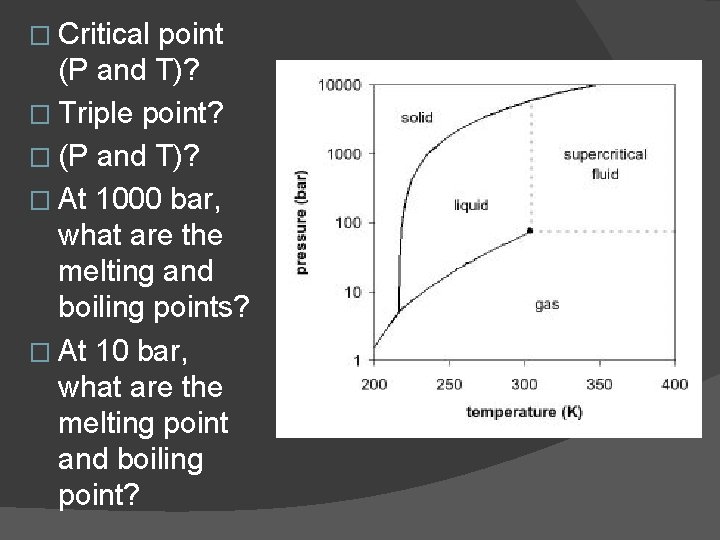
� Critical point (P and T)? � Triple point? � (P and T)? � At 1000 bar, what are the melting and boiling points? � At 10 bar, what are the melting point and boiling point?

Thermal Equilibrium Problems �

Example �

Example � You pee on an icecube at 0 degrees Fahrenheit (m= 11 grams) in a thermally insulated cup with a liquid that has a specific heat very similar to water and is at a temperature of 40 C. How many m. L will you have to unload in order to completely melt the icecube at thermal equilibrium?

Conduction Problem �

Intensity �

� A 1200 Watt speaker is in the center of a 10 x 10 meter square room on a pedestal, at the same height as your ear. If you’re standing on the perimeter of the room, and assuming that your ear has an area of 1 cm 2, �What is the intensity of the speaker where your ear is at? �What is the power delivered to your ear, in Watts? �What is the power delivered to your ear, in decibels? �If you double the distance between you and the speaker, what will the new intensity in decibels be?

Heat Transfer � How much heat energy per second is lost due to radiation by a 40 degree Celsius basketball (r=6 in. ) inside a 20 degree Celsius room? Assume blackbody radiation.

Thermal Expansion �

Do I need to convert to Kelvin? �

Situation Must I convert to Kelvin, or can I use Celsius? Celsius OKAY. Kelvin OKAY. Celsius NOT OKAY. Kelvin OKAY.

Carnot Cycle � What is the efficiency of the Carnot Cycle if the TH=30 °C, Tc=-100°C?

� Two sides of a room contain gas at a different temperature. After 20 minutes the gases have mixed. This is irreversible. (T/F) � You touch a warm pot with your hand. The heat flow that occurs is reversible. (T/F) � You can convert 30 J of work into 30 J of heat and leave everything else the same. (T/F) � You can convert 30 J of heat into 30 J of work and leave everything else the same. (T/F)

� An Otto cycle expands gas from 30 m 3 to 50 m 3. �What is the efficiency if monatomic oxygen is used? �What is the efficiency if diatomic oxygen is used?

� You have 5 kg of CO 2 (m=48 g/mol) in a box of volume 4 m 3 at a pressure of 50 k. Pa. What is the temperature of the gas?

� A monatomic gas increases triples in volume during an adiabatic process. If the initial temperature is 50 degrees Celsius, what is the final temperature? � You do 50 J of work on a system and dump 30 J of heat in; what is the change in internal energy of the system?

� A gas expands at constant pressure from a volume of 10 m 3 to a volume of 30 m 3 at a pressure of 20 k. Pa. What is the work done on the gas?

� Two moles of an ideal diatomic gas have an initial pressure of 1. 00 atm and initial volume of 40. 0 L. The gas molecules can translate and rotate but not vibrate. The gas is compressed at constant pressure to a new volume of 30. 0 L. The change in the internal energy of the gas during this process is ____ L·atm.

� Consider an Otto cycle where 20 J of energy go in and 10 J of energy go out. What is the efficiency? � A refrigerator operating in a cycle has a coefficient of performance of 6. 00. To remove 9. 00 J of heat from inside the refrigerator to the outside per cycle requires ____ J of work to be done on the refrigerator per cycle.


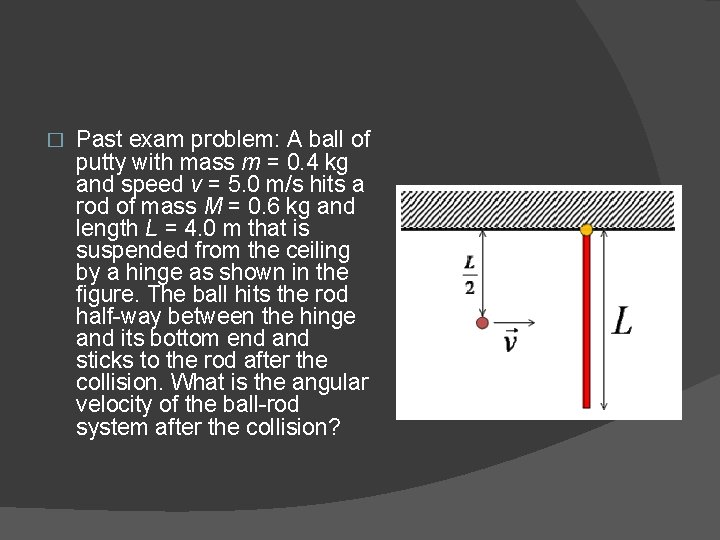
� Past exam problem: A ball of putty with mass m = 0. 4 kg and speed v = 5. 0 m/s hits a rod of mass M = 0. 6 kg and length L = 4. 0 m that is suspended from the ceiling by a hinge as shown in the figure. The ball hits the rod half-way between the hinge and its bottom end and sticks to the rod after the collision. What is the angular velocity of the ball-rod system after the collision?