PHY 113 C General Physics I 11 AM








- Slides: 29

PHY 113 C General Physics I 11 AM – 12: 15 PM MWF Olin 101 1. 2. 3. 4. Plan for Lecture 23: Chapter 22: Heat engines Thermodynamic cycles; work and heat efficiency Carnot cycle Otto cycle; diesel cycle Brief comments on entropy 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 1

11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 2

Comment about Exam 3: • Part I – take home portion (1 problem): available Thursday 11/21/2013 after class; must be turned in before Part II – in-class portion (3 problems): Tuesday 11/25/2013 • Some special arrangements for early exams have been (or will be) arranged by prior agreement • Of course, all sections of the exam are to be taken under the guidelines of the honor code 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 3

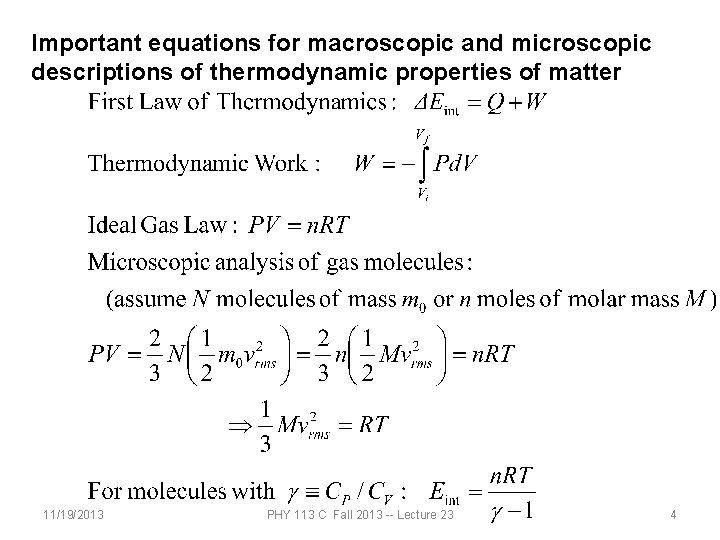
Important equations for macroscopic and microscopic descriptions of thermodynamic properties of matter 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 4

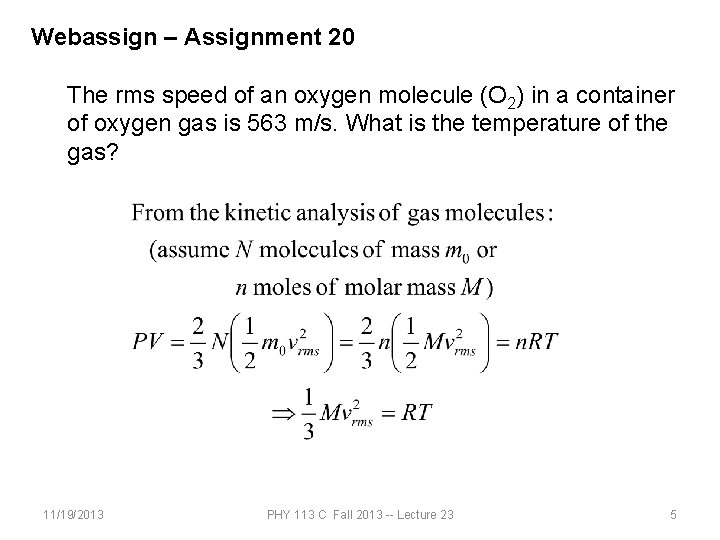
Webassign – Assignment 20 The rms speed of an oxygen molecule (O 2) in a container of oxygen gas is 563 m/s. What is the temperature of the gas? 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 5

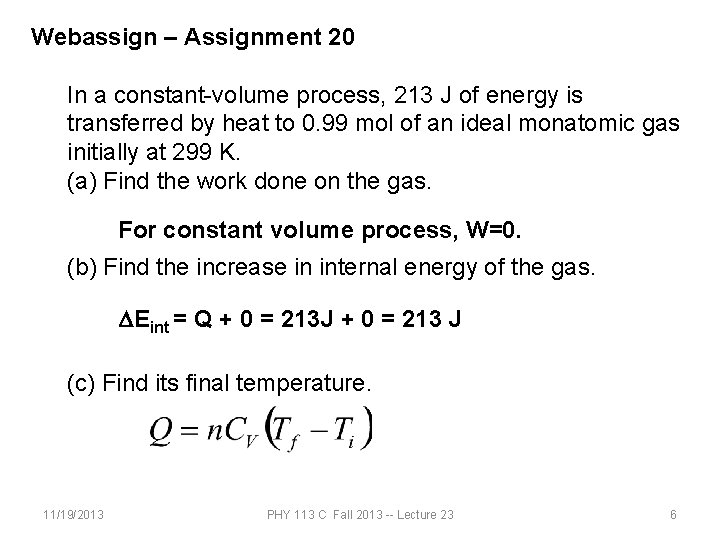
Webassign – Assignment 20 In a constant-volume process, 213 J of energy is transferred by heat to 0. 99 mol of an ideal monatomic gas initially at 299 K. (a) Find the work done on the gas. For constant volume process, W=0. (b) Find the increase in internal energy of the gas. DEint = Q + 0 = 213 J + 0 = 213 J (c) Find its final temperature. 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 6

Webassign – Assignment 20 A 2. 00 -mol sample of a diatomic ideal gas expands slowly and adiabatically from a pressure of 5. 06 atm and a volume of 12. 2 L to a final volume of 29. 6 L. (a) What is the final pressure of the gas? (b) What are the initial and final temperatures? (c) Find Q for the gas during this process. (d) Find ΔEint for the gas during this process. (e) Find W for the gas during this process. 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 7

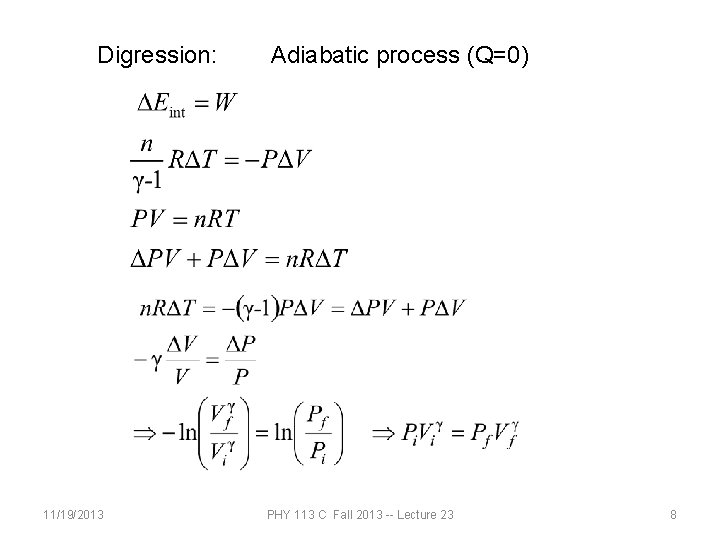
Digression: 11/19/2013 Adiabatic process (Q=0) PHY 113 C Fall 2013 -- Lecture 23 8

Webassign – Assignment 20 A 2. 00 -mol sample of a diatomic ideal gas expands slowly and adiabatically from a pressure of 5. 06 atm and a volume of 12. 2 L to a final volume of 29. 6 L. For diatomic ideal gas: g = 1. 4 (a) What is the final pressure of the gas? b) What are the initial and final temperatures? PV=n. RT c) Find Q for the gas during this process. Q=0 d) Find ΔEint for the gas during this process. ΔEint=W e) Find W for the gas during this process. 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 9

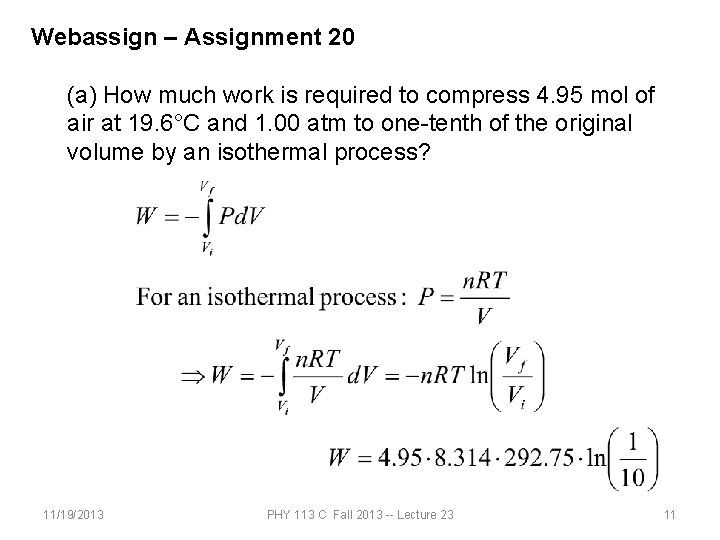
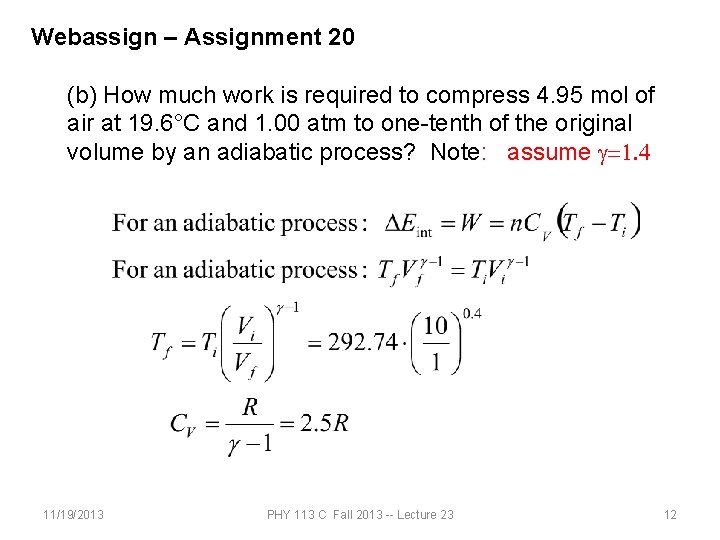
Webassign – Assignment 20 (a) How much work is required to compress 4. 95 mol of air at 19. 6°C and 1. 00 atm to one-tenth of the original volume by an isothermal process? (b) How much work is required to produce the same compression in an adiabatic process? (c) What is the final pressure in part (a)? (d) What is the final pressure in part (b)? 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 10

Webassign – Assignment 20 (a) How much work is required to compress 4. 95 mol of air at 19. 6°C and 1. 00 atm to one-tenth of the original volume by an isothermal process? 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 11

Webassign – Assignment 20 (b) How much work is required to compress 4. 95 mol of air at 19. 6°C and 1. 00 atm to one-tenth of the original volume by an adiabatic process? Note: assume g=1. 4 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 12

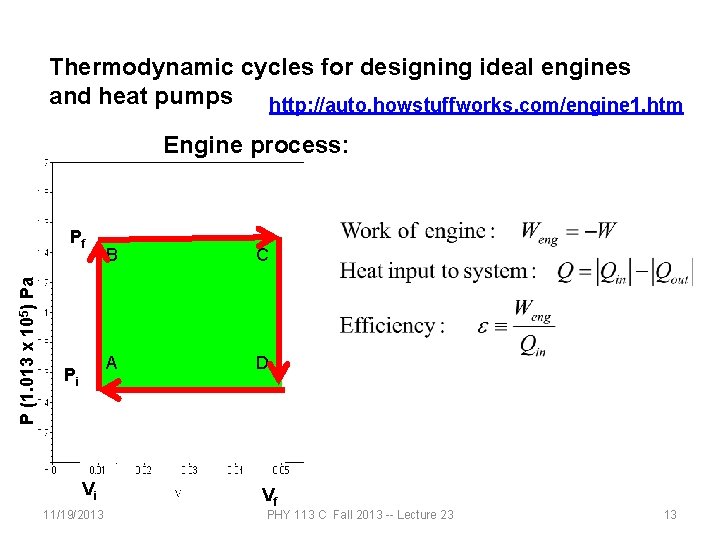
Thermodynamic cycles for designing ideal engines and heat pumps http: //auto. howstuffworks. com/engine 1. htm Engine process: P (1. 013 x 105) Pa Pf Pi Vi 11/19/2013 B C A D Vf PHY 113 C Fall 2013 -- Lecture 23 13

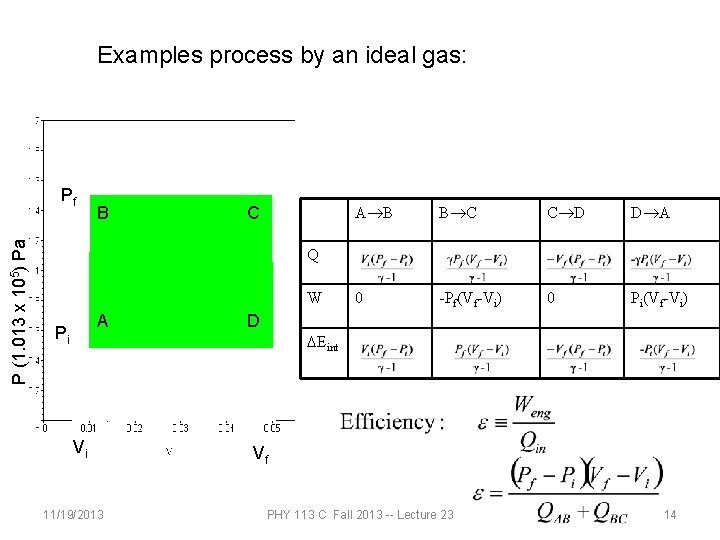
Examples process by an ideal gas: P (1. 013 x 105) Pa Pf B C A®B B®C C®D D®A 0 -Pf(Vf-Vi) 0 Pi(Vf-Vi) Q W A Pi Vi 11/19/2013 D DEint Vf PHY 113 C Fall 2013 -- Lecture 23 14

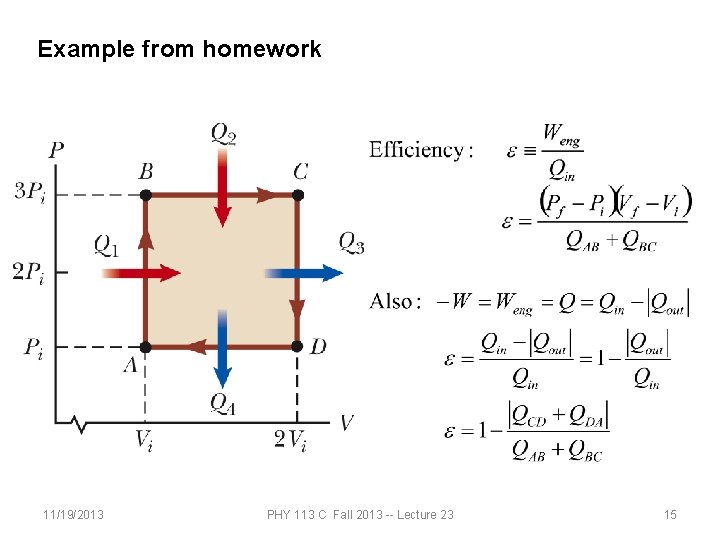
Example from homework 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 15

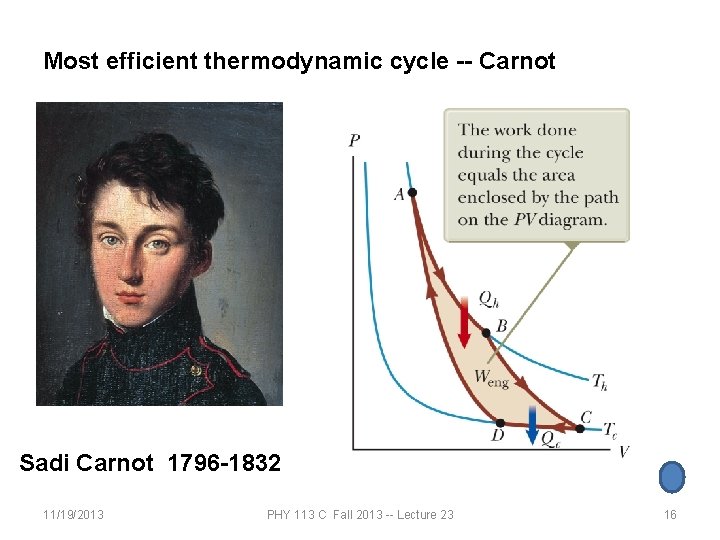
Most efficient thermodynamic cycle -- Carnot Sadi Carnot 1796 -1832 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 16

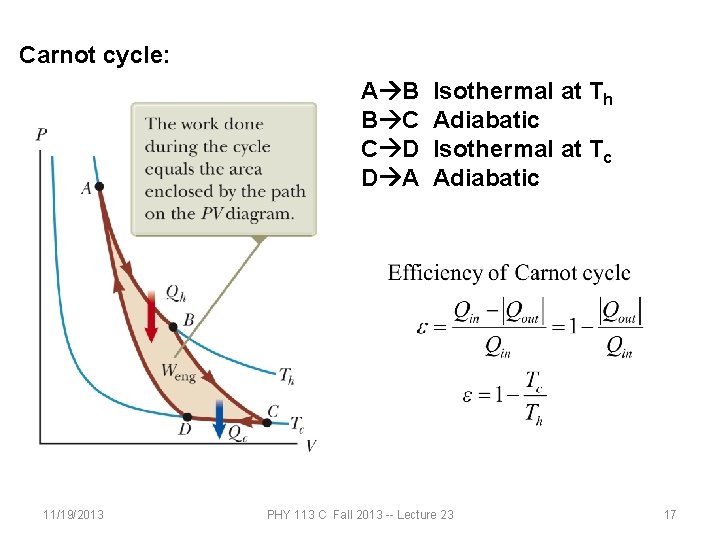
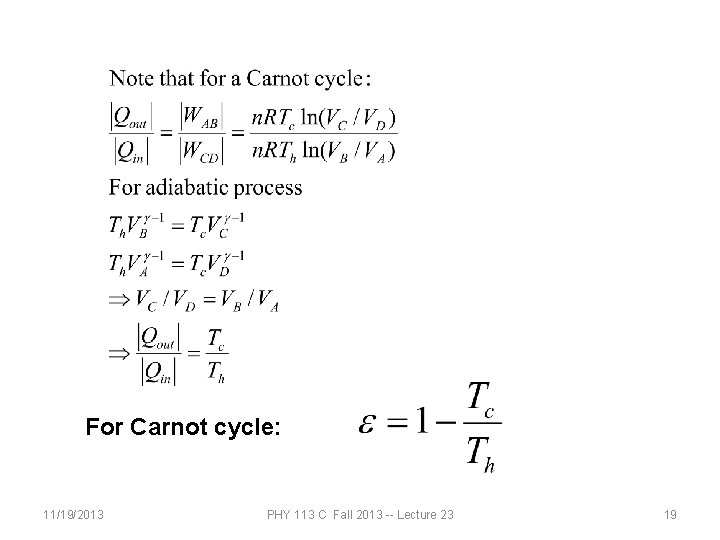
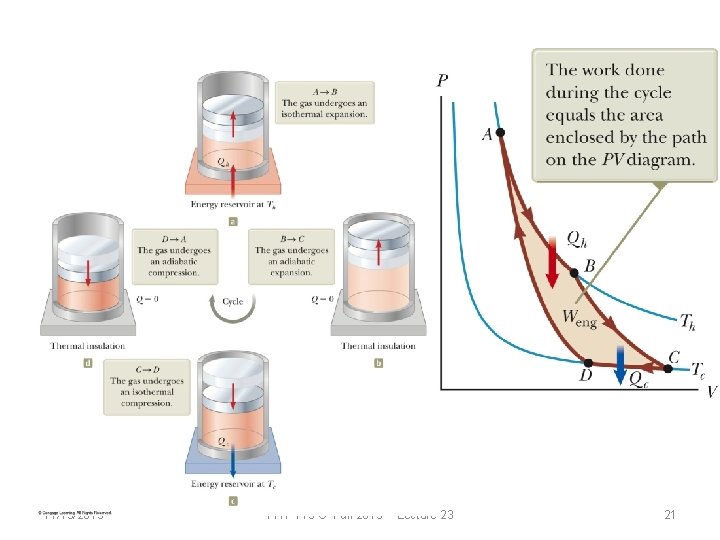
Carnot cycle: A B B C C D D A 11/19/2013 Isothermal at Th Adiabatic Isothermal at Tc Adiabatic PHY 113 C Fall 2013 -- Lecture 23 17

iclicker exercise: We discussed the efficiency of an engine as Is this result A. Special to the Carnot cycle B. General to all ideal thermodynamic cycles iclicker exercise: We discussed the efficiency of an engine running with hot and cold reservoirs as Is this result A. Special to the Carnot cycle B. General to all ideal thermodynamic cycles 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 18

For Carnot cycle: 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 19

iclicker exercise: Why should we care about the Carnot cycle? A. We shouldn’t B. It approximately models some heating and cooling technologies C. It provides insight into anothermodynamic variable -- entropy 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 20

11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 21

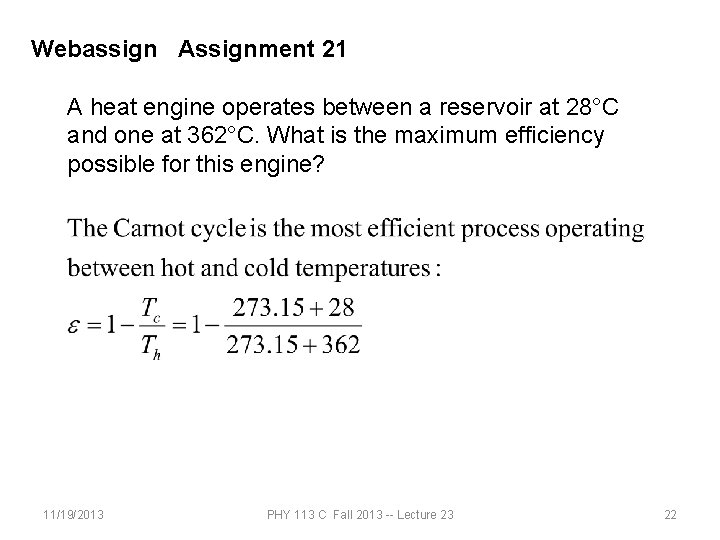
Webassign Assignment 21 A heat engine operates between a reservoir at 28°C and one at 362°C. What is the maximum efficiency possible for this engine? 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 22

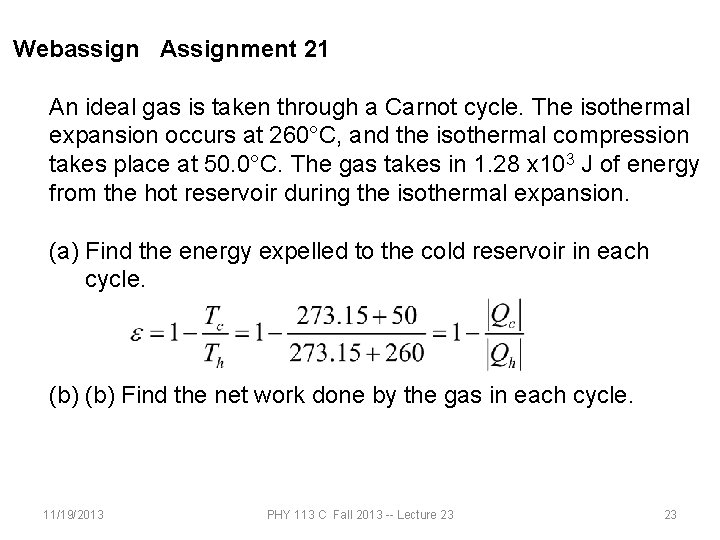
Webassign Assignment 21 An ideal gas is taken through a Carnot cycle. The isothermal expansion occurs at 260°C, and the isothermal compression takes place at 50. 0°C. The gas takes in 1. 28 x 103 J of energy from the hot reservoir during the isothermal expansion. (a) Find the energy expelled to the cold reservoir in each cycle. (b) Find the net work done by the gas in each cycle. 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 23

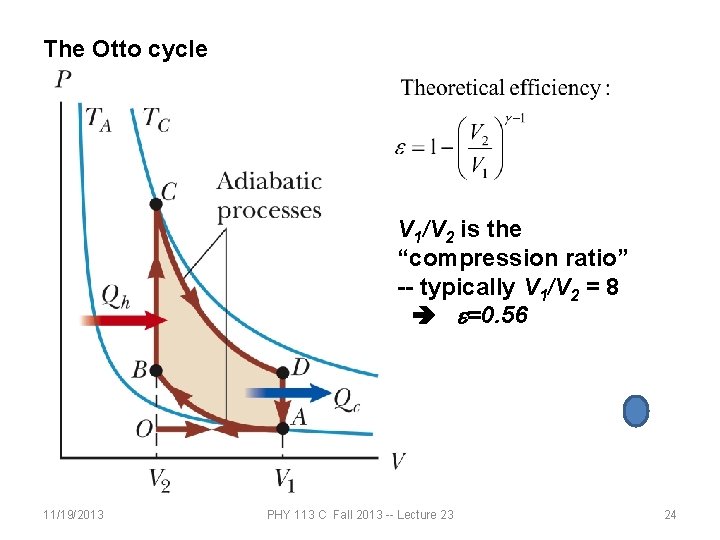
The Otto cycle V 1/V 2 is the “compression ratio” -- typically V 1/V 2 = 8 e=0. 56 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 24

11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 25

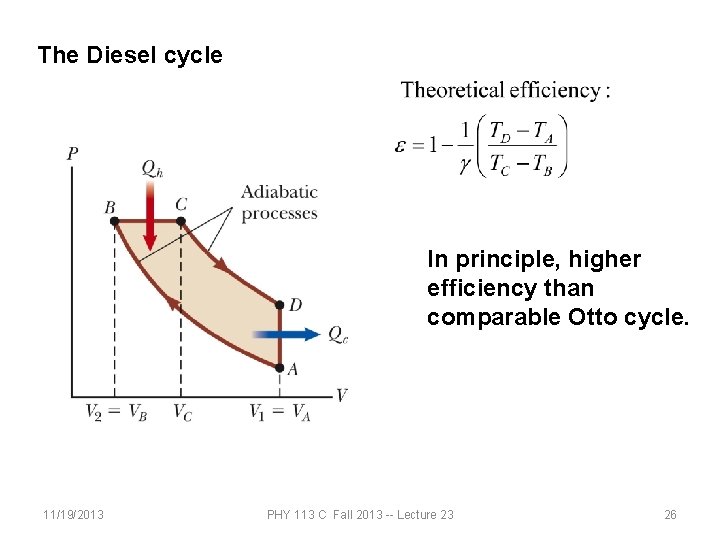
The Diesel cycle In principle, higher efficiency than comparable Otto cycle. 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 26

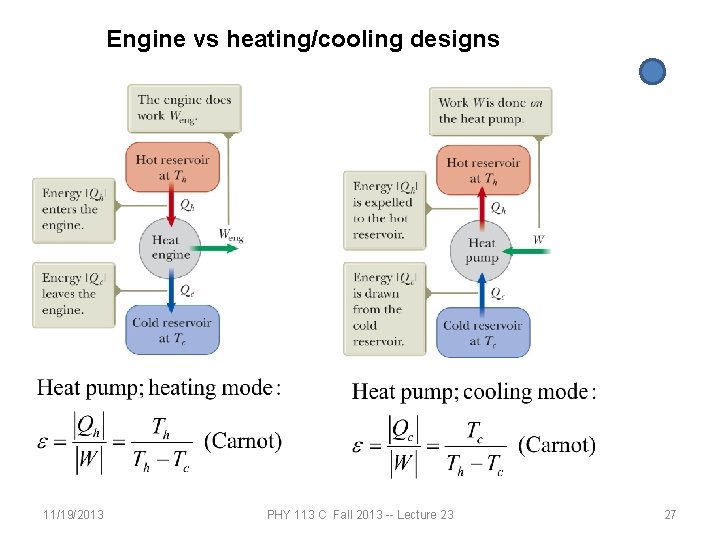
Engine vs heating/cooling designs 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 27

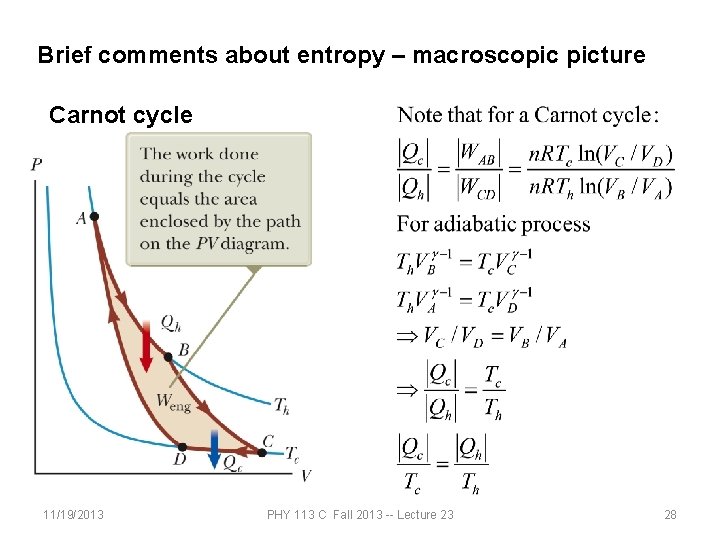
Brief comments about entropy – macroscopic picture Carnot cycle 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 28

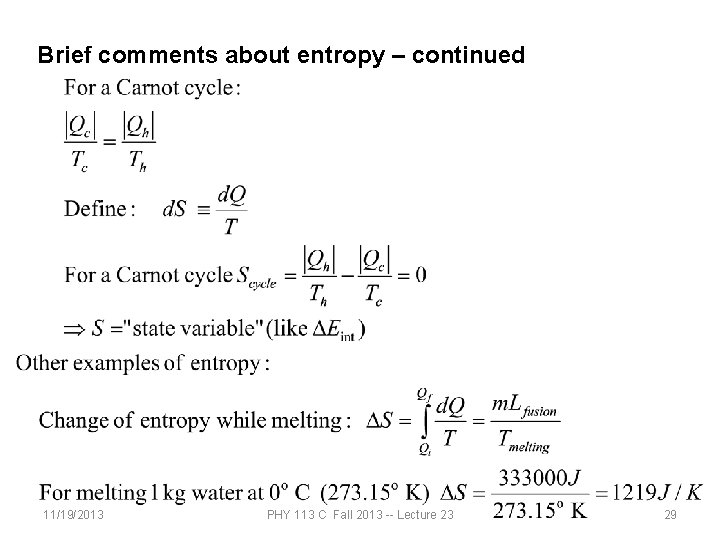
Brief comments about entropy – continued 11/19/2013 PHY 113 C Fall 2013 -- Lecture 23 29