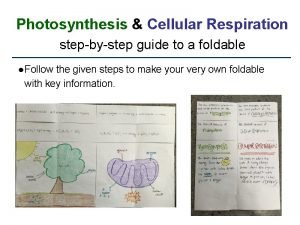
Photosynthesis and Cellular Respiration Outline I Photosynthesis A



- Slides: 28

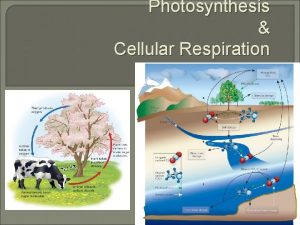
Photosynthesis and Cellular Respiration

Outline I. Photosynthesis A. Introduction B. Reactions II. Cellular Respiration A. Introduction B. Reactions

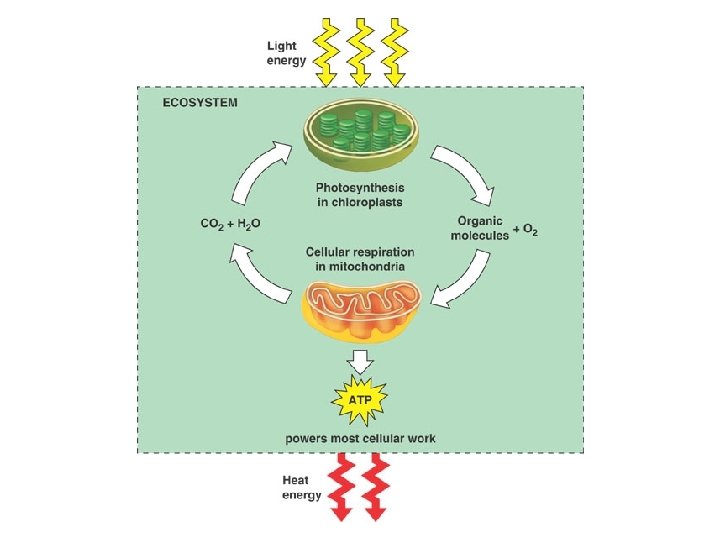
Photosynthesis l l Method of converting sun energy into chemical energy usable by cells Autotrophs: self feeders, organisms capable of making their own food – – Photoautotrophs: use sun energy e. g. plants photosynthesis-makes organic compounds (glucose) from light Chemoautotrophs: use chemical energy e. g. bacteria that use sulfide or methane chemosynthesismakes organic compounds from chemical energy contained in sulfide or methane

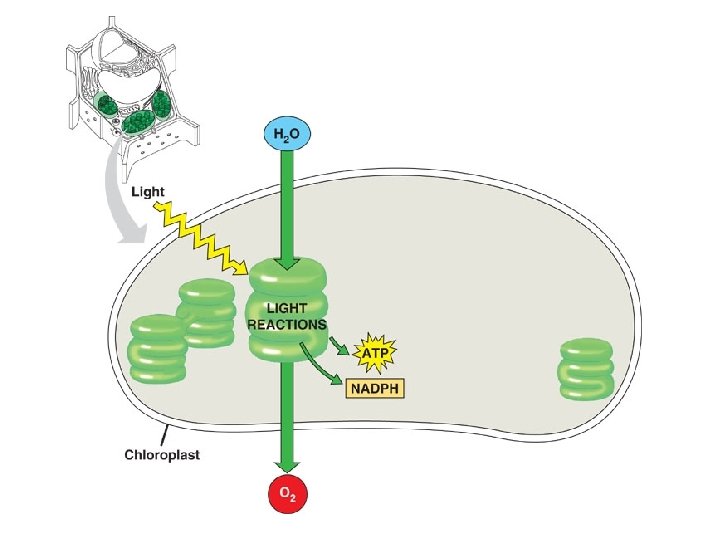
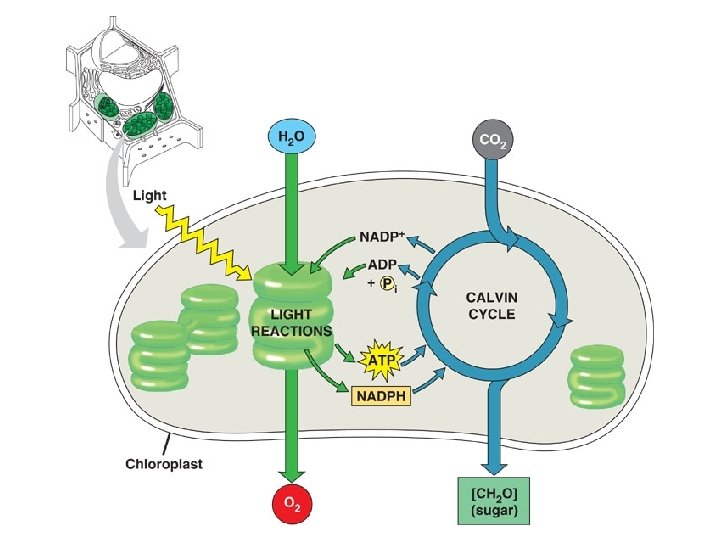
Photosynthesis l Photosynthesis takes place in specialized structures inside plant cells called chloroplasts – Light absorbing pigment molecules e. g. chlorophyll

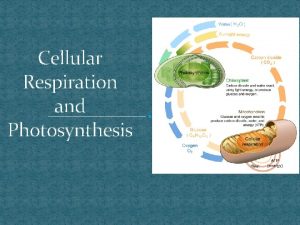
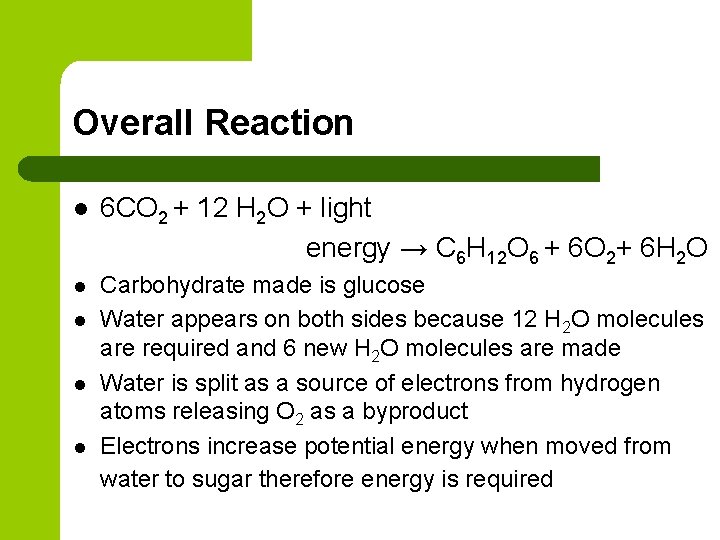
Overall Reaction l 6 CO 2 + 12 H 2 O + light energy → C 6 H 12 O 6 + 6 O 2+ 6 H 2 O l Carbohydrate made is glucose Water appears on both sides because 12 H 2 O molecules are required and 6 new H 2 O molecules are made Water is split as a source of electrons from hydrogen atoms releasing O 2 as a byproduct Electrons increase potential energy when moved from water to sugar therefore energy is required l l l

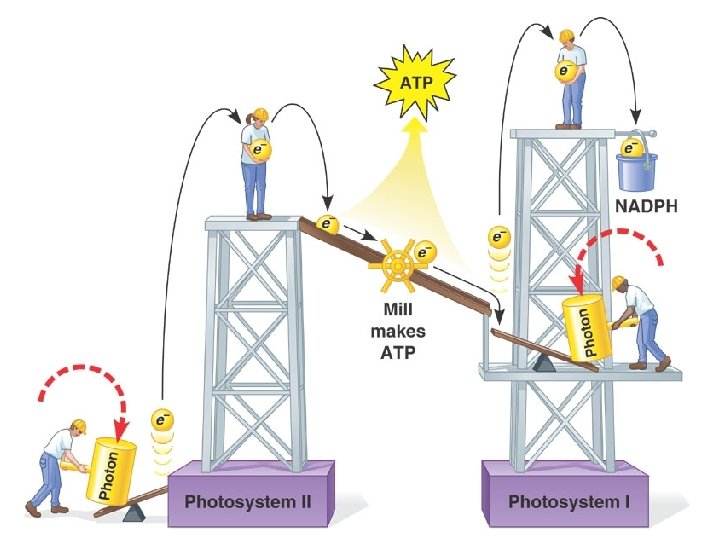
Light-dependent Reactions l Overview: light energy is absorbed by chlorophyll molecules-this light energy excites electrons and boosts them to higher energy levels. They are trapped by electron acceptor molecules that are poised at the start of a neighboring transport system. The electrons “fall” to a lower energy state, releasing energy that is harnessed to make ATP

Energy Shuttling l l Recall ATP: cellular energy-nucleotide based molecule with 3 phosphate groups bonded to it, when removing the third phosphate group, lots of energy liberated= superb molecule for shuttling energy around within cells. Other energy shuttles-coenzymes (nucleotide based molecules): move electrons and protons around within the cell NADP+, NADPH NAD+, NADP FAD, FADH 2

Light-dependent Reactions l l Photosystem: light capturing unit, contains chlorophyll, the light capturing pigment Electron transport system: sequence of electron carrier molecules that shuttle electrons, energy released to make ATP Electrons in chlorophyll must be replaced so that cycle may continue-these electrons come from water molecules, Oxygen is liberated from the light reactions Light reactions yield ATP and NADPH used to fuel the reactions of the Calvin cycle (light independent or dark reactions)



Calvin Cycle (light independent or “dark” reactions) l l l ATP and NADPH generated in light reactions used to fuel the reactions which take CO 2 and break it apart, then reassemble the carbons into glucose. Called carbon fixation: taking carbon from an inorganic molecule (atmospheric CO 2) and making an organic molecule out of it (glucose) Simplified version of how carbon and energy enter the food chain


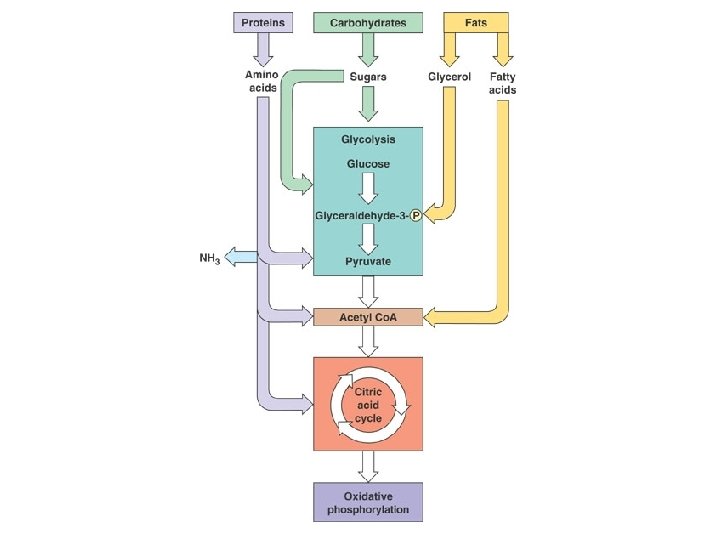
Harvesting Chemical Energy l l So we see how energy enters food chains (via autotrophs) we can look at how organisms use that energy to fuel their bodies. Plants and animals both use products of photosynthesis (glucose) for metabolic fuel Heterotrophs: must take in energy from outside sources, cannot make their own e. g. animals When we take in glucose (or other carbs), proteins, and fats-these foods don’t come to us the way our cells can use them

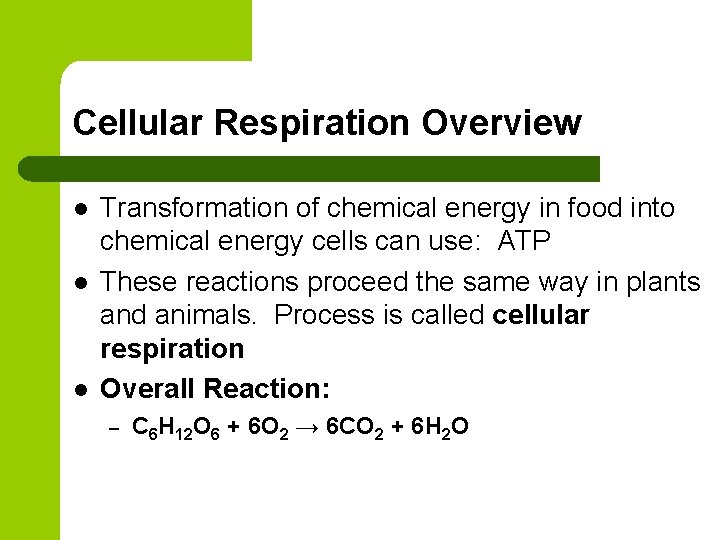
Cellular Respiration Overview l l l Transformation of chemical energy in food into chemical energy cells can use: ATP These reactions proceed the same way in plants and animals. Process is called cellular respiration Overall Reaction: – C 6 H 12 O 6 + 6 O 2 → 6 CO 2 + 6 H 2 O

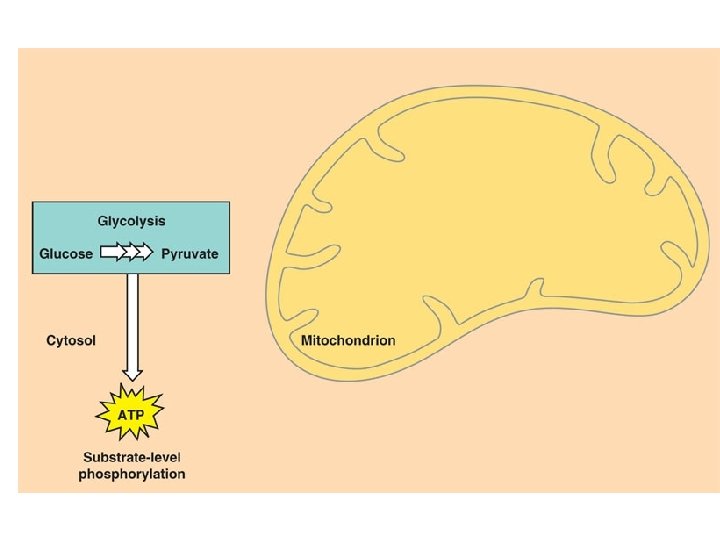
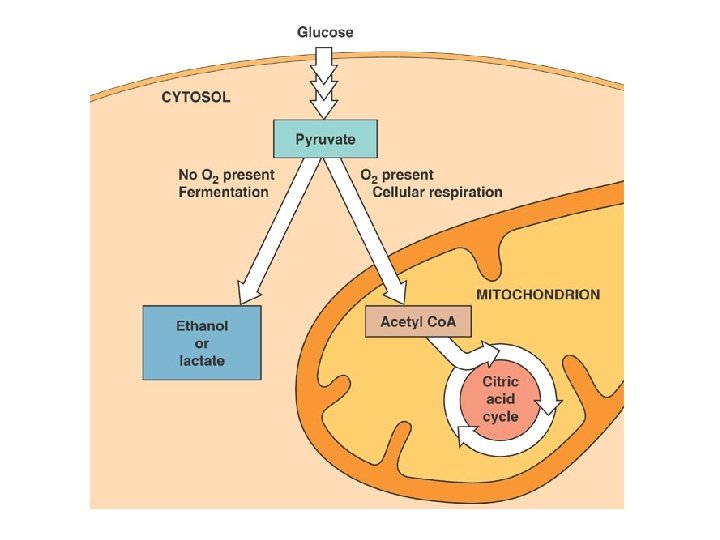
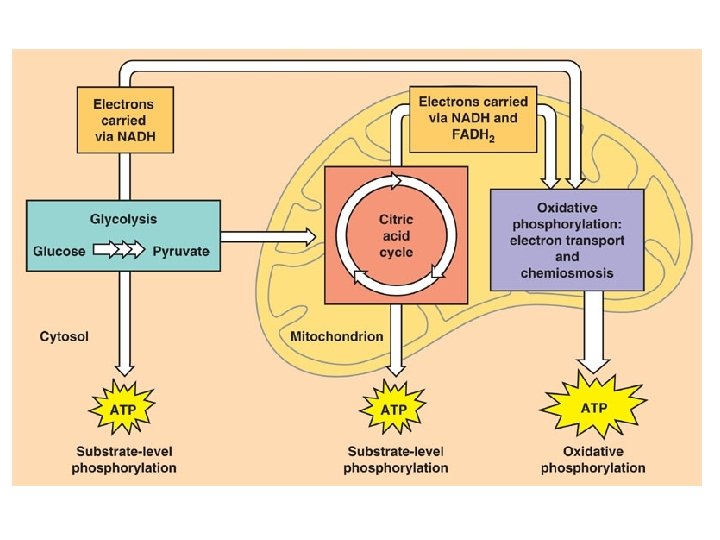
Cellular Respiration Overview l l Breakdown of glucose begins in the cytoplasm: the liquid matrix inside the cell At this point life diverges into two forms and two pathways – – Anaerobic cellular respiration (aka fermentation) Aerobic cellular respiration

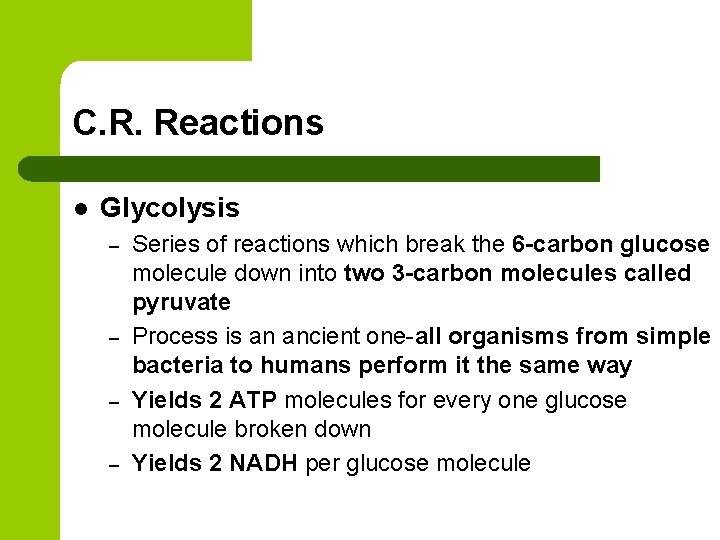
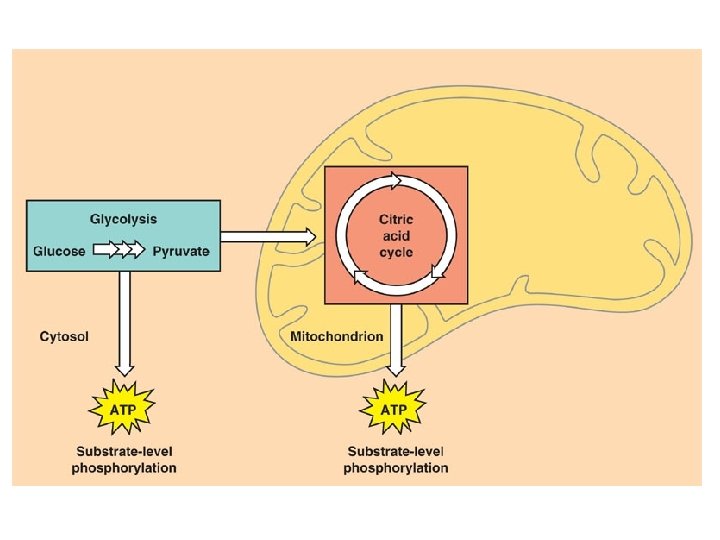
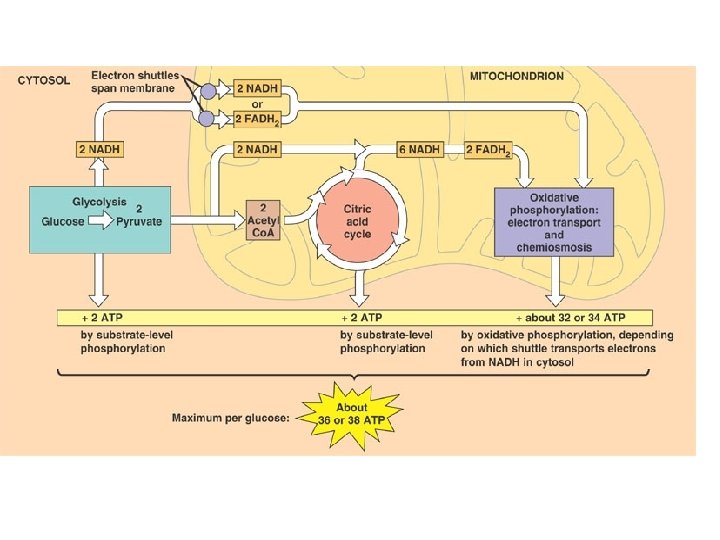
C. R. Reactions l Glycolysis – – Series of reactions which break the 6 -carbon glucose molecule down into two 3 -carbon molecules called pyruvate Process is an ancient one-all organisms from simple bacteria to humans perform it the same way Yields 2 ATP molecules for every one glucose molecule broken down Yields 2 NADH per glucose molecule


Anaerobic Cellular Respiration l Some organisms thrive in environments with little or no oxygen – l l l Marshes, bogs, gut of animals, sewage treatment ponds No oxygen used= ‘an’aerobic Results in no more ATP, final steps in these pathways serve ONLY to regenerate NAD+ so it can return to pick up more electrons and hydrogens in glycolysis. End products such as ethanol and CO 2 (single cell fungi (yeast) in beer/bread) or lactic acid (muscle cells)


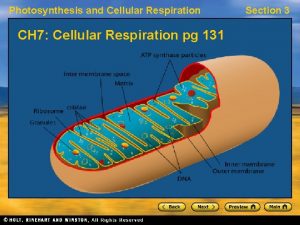
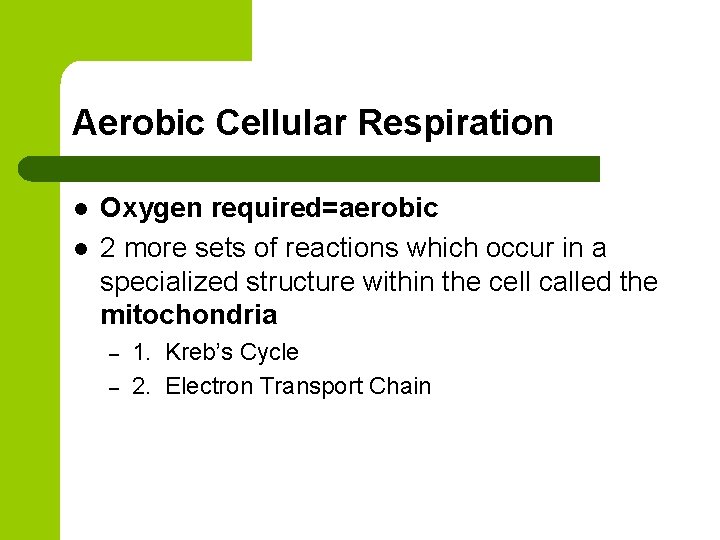
Aerobic Cellular Respiration l l Oxygen required=aerobic 2 more sets of reactions which occur in a specialized structure within the cell called the mitochondria – – 1. Kreb’s Cycle 2. Electron Transport Chain

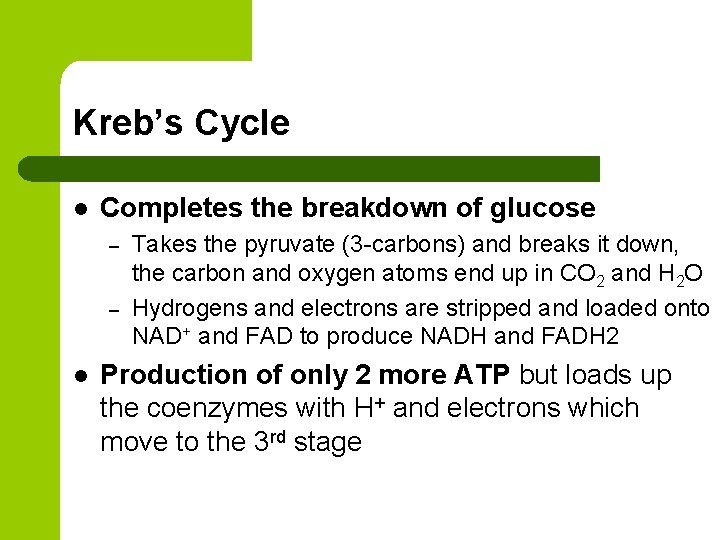
Kreb’s Cycle l Completes the breakdown of glucose – – l Takes the pyruvate (3 -carbons) and breaks it down, the carbon and oxygen atoms end up in CO 2 and H 2 O Hydrogens and electrons are stripped and loaded onto NAD+ and FAD to produce NADH and FADH 2 Production of only 2 more ATP but loads up the coenzymes with H+ and electrons which move to the 3 rd stage


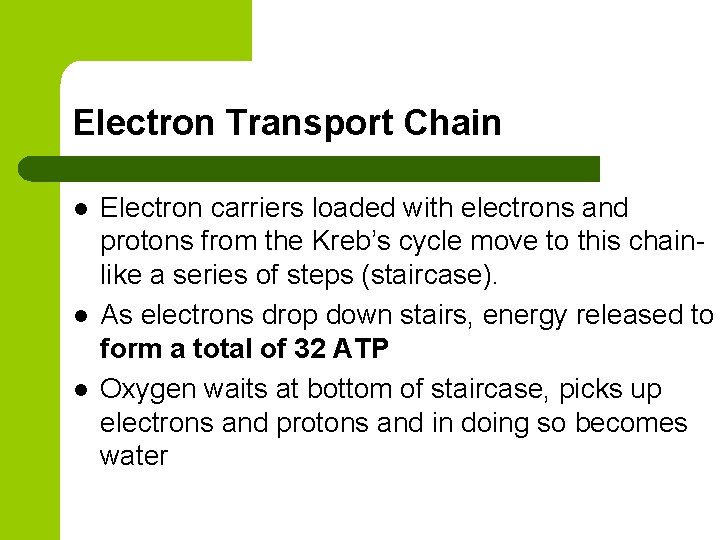
Electron Transport Chain l l l Electron carriers loaded with electrons and protons from the Kreb’s cycle move to this chainlike a series of steps (staircase). As electrons drop down stairs, energy released to form a total of 32 ATP Oxygen waits at bottom of staircase, picks up electrons and protons and in doing so becomes water


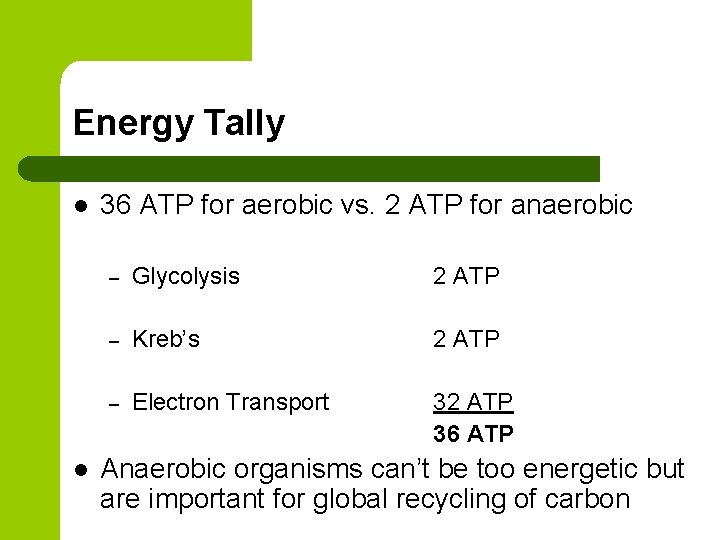
Energy Tally l l 36 ATP for aerobic vs. 2 ATP for anaerobic – Glycolysis 2 ATP – Kreb’s 2 ATP – Electron Transport 32 ATP 36 ATP Anaerobic organisms can’t be too energetic but are important for global recycling of carbon



 Complimentary processes
Complimentary processes Equation of cellular respiration
Equation of cellular respiration What's the equation for cellular respiration
What's the equation for cellular respiration Function of cellular respiration
Function of cellular respiration Where does cellular respiration take place
Where does cellular respiration take place Photosynthesis and cellular respiration foldable
Photosynthesis and cellular respiration foldable Where does the oxygen that we breathe come from edpuzzle
Where does the oxygen that we breathe come from edpuzzle Photosynthesis and cellular respiration
Photosynthesis and cellular respiration Photosynthesis and cellular respiration virtual lab
Photosynthesis and cellular respiration virtual lab Photosynthesis and cellular respiration jeopardy
Photosynthesis and cellular respiration jeopardy Photosynthesis or cellular respiration
Photosynthesis or cellular respiration Brain warmer
Brain warmer Cellular respiration redox
Cellular respiration redox What is the correct equation for cellular respiration?
What is the correct equation for cellular respiration? Chemiosmosis steps
Chemiosmosis steps Types of respiration
Types of respiration Was ist fermentieren
Was ist fermentieren Why is cellular respiration important
Why is cellular respiration important Chemical equation for cellular respiration
Chemical equation for cellular respiration Amloplast
Amloplast Photosynthesis recipe card
Photosynthesis recipe card What happens during glycolysis
What happens during glycolysis Overview of cellular respiration
Overview of cellular respiration Overview of aerobic respiration
Overview of aerobic respiration Overview of cellular respiration
Overview of cellular respiration Overview of cellular respiration
Overview of cellular respiration Lab bench cellular respiration
Lab bench cellular respiration What is the word equation for cellular respiration
What is the word equation for cellular respiration Higher human biology cellular respiration
Higher human biology cellular respiration