Photoionization Spectra of Atomic Sulfur 1 D in

![LS Coupling for Sulfur Atom S [Ne]3 s 23 p 4 configuration 1 S LS Coupling for Sulfur Atom S [Ne]3 s 23 p 4 configuration 1 S](https://slidetodoc.com/presentation_image_h2/c4aa86799fa658bf05fe938084c5acf5/image-2.jpg)


![Low Energy nd Series Er / cm-1 Elevel /cm-1 assignment n*[3/2] n*[5/2] μ[3/2] μ[5/2] Low Energy nd Series Er / cm-1 Elevel /cm-1 assignment n*[3/2] n*[5/2] μ[3/2] μ[5/2]](https://slidetodoc.com/presentation_image_h2/c4aa86799fa658bf05fe938084c5acf5/image-22.jpg)
![New Series from jcl Coupling Er / cm-1 Elevel /cm-1 assignment n*[3/2] n*[5/2] μ[3/2] New Series from jcl Coupling Er / cm-1 Elevel /cm-1 assignment n*[3/2] n*[5/2] μ[3/2]](https://slidetodoc.com/presentation_image_h2/c4aa86799fa658bf05fe938084c5acf5/image-23.jpg)


- Slides: 25

Photoionization Spectra of Atomic Sulfur 1 D in Transitions to Autoionizing Rydberg States in the Region 75800 -89500 cm-1 (9. 4 -11. 1 e. V) Wan-Chun Pan Department of Chemistry, National Tsing Hua University National Synchrotron Radiation Research Center
![LS Coupling for Sulfur Atom S Ne3 s 23 p 4 configuration 1 S LS Coupling for Sulfur Atom S [Ne]3 s 23 p 4 configuration 1 S](https://slidetodoc.com/presentation_image_h2/c4aa86799fa658bf05fe938084c5acf5/image-2.jpg)
LS Coupling for Sulfur Atom S [Ne]3 s 23 p 4 configuration 1 S 1 S 1 D 1 D 3 P 3 P 2 P 0 2 D 0 S+ [Ne]3 s 23 p 3 configuration 3 P 2 4 S 0 is the ground state of S, and 4 S 0 of S+ 3 P 3 P 0 2 0 1 2 2 P 0 2 D 0 3/2 1/2 5/2 3/2 4 S 0

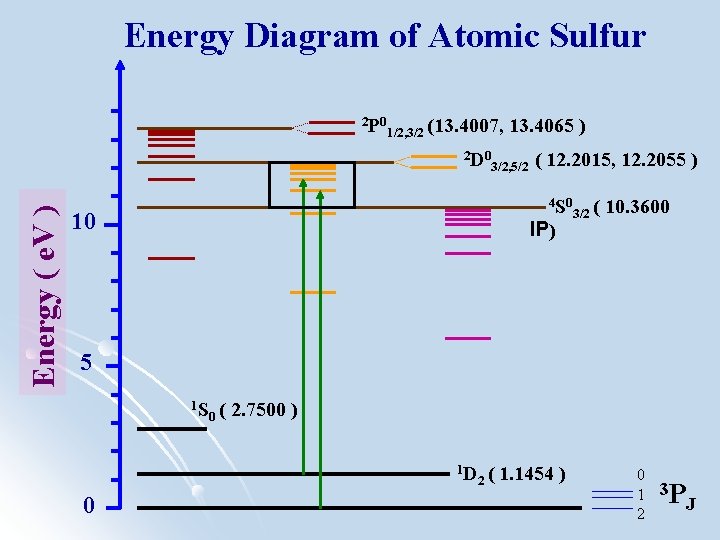
Energy Diagram of Atomic Sulfur 2 P 0 1/2, 3/2 (13. 4007, Energy ( e. V ) 2 D 0 13. 4065 ) 3/2, 5/2 ( 12. 2015, 12. 2055 ) 4 S 0 10 IP) 3/2 ( 10. 3600 5 1 S 0( 2. 7500 ) 1 D 0 2( 1. 1454 ) 0 1 3 2 PJ

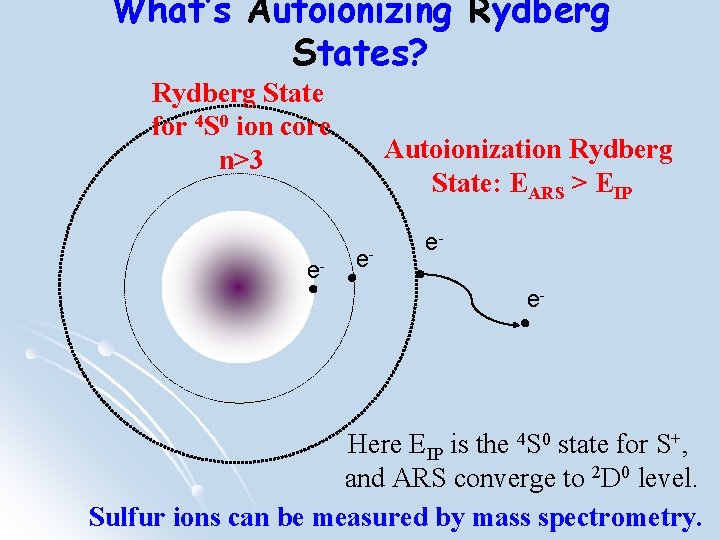
What’s Autoionizing Rydberg States? Rydberg State for 4 S 0 ion core n>3 e- Autoionization Rydberg State: EARS > EIP e- ee- Here EIP is the 4 S 0 state for S+, and ARS converge to 2 D 0 level. Sulfur ions can be measured by mass spectrometry.

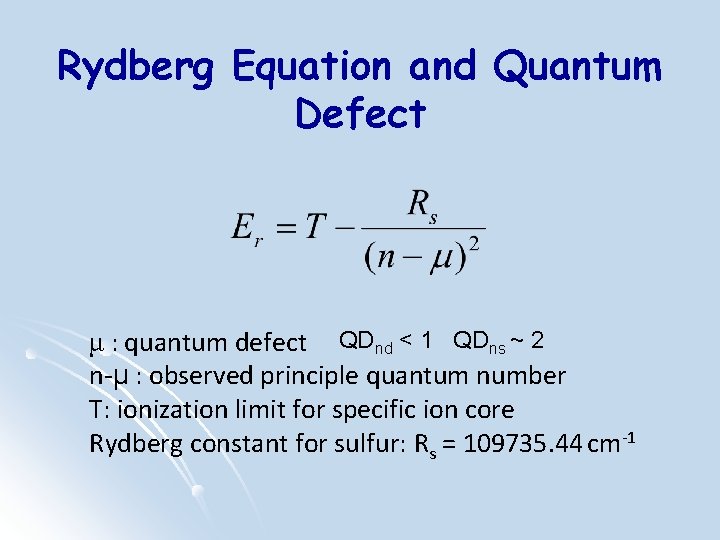
Rydberg Equation and Quantum Defect μ : quantum defect QDnd < 1 QDns ~ 2 n-μ : observed principle quantum number T: ionization limit for specific ion core Rydberg constant for sulfur: Rs = 109735. 44 cm-1

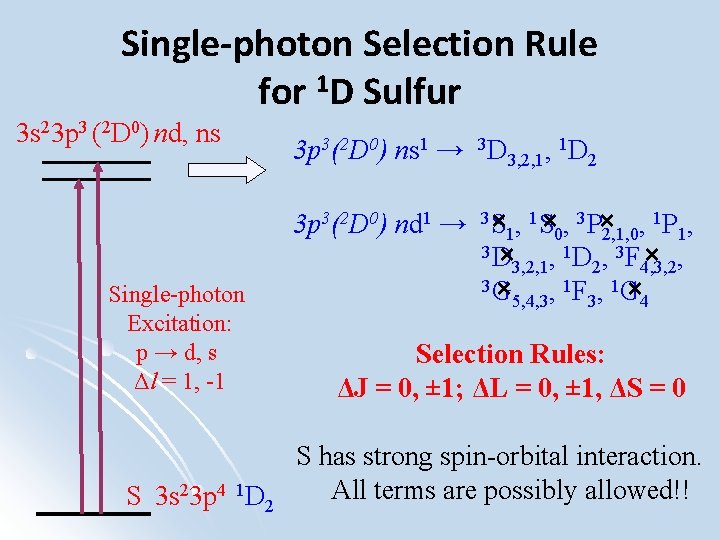
Single-photon Selection Rule for 1 D Sulfur 3 s 23 p 3 (2 D 0) nd, ns Single-photon Excitation: p → d, s Δl = 1, -1 S 3 s 23 p 4 1 D 2 3 p 3(2 D 0) ns 1 → 3 D 3, 2, 1, 1 D 2 1 3 p 3(2 D 0) nd 1 → 3× S 1, 1 S× 0, 3 P× 2, 1, 0, P 1, 3 D × 3, 2, 1, 1 D 2, 3 F 4, 3, 2 × , 3 G × 5, 4, 3, 1 F 3, 1 G× 4 Selection Rules: ΔJ = 0, ± 1; ΔL = 0, ± 1, ΔS = 0 S has strong spin-orbital interaction. All terms are possibly allowed!!

Review l S. T. Gibson, J. P. Greene, B. Ruscic, and J. Berkowitz, J. Phys. B 19, 2825 (1986) 3 s 23 p 4 3 P 2 ―> 3 s 23 p 3(2 D 0) ns 3 D 3 s 23 p 4 3 P 2 ―> 3 s 23 p 3(2 D 0) nd 3 P 1, 2, 3 S, 3 D l J. Huang, D. D. Xu, A. Stuchebrukhov, and W. M. Jackson, J. Chem. Phys. 122, 144321 (2005) X. L. Yang, J. G. Zhou, B. Jones, C. Y. Ng, and W. M. Jackson, J. Chem. Phys. 128, 084303 (2008) 3 s 23 p 4 1 D 2 ―> 3 s 23 p 3(2 D 0) ns 3 D 3, 2, 1, 1 D 2 l 3 s 23 p 4 1 D 2 ―> 3 s 23 p 3(2 D 0) nd 3 S 1, 1 S 0, 3 P 2, 1, 1 P 1, 3 D 3, 2, 1, 1 D 2, 3 F Energy range: < 84950 cm-1 1 F , , 4, 3, 2 3

ü Source of 1 D S: CS 2 + hν 193 nm → CS (1Σ+ ) + S (3 P) → CS (1Σ+ ) + S (1 D) ü This work: Detection of ARS from 1 D using synchrotron radiation • Advantages of Synchrotron Radiation → High flux in VUV region → Wide range and continuously scan in 8 -40 e. V • Spectral Resolution of U 9 CGM → E/ΔE = 20000 at 10 e. V, resolution 0. 5 me. V(~ 4 cm-1) → Best resolution: E/ΔE = 60000 (~ 1. 33 cm-1)

Beamline U 9 CGM at NSRRC Beamline Status Monochromater

Experimental System

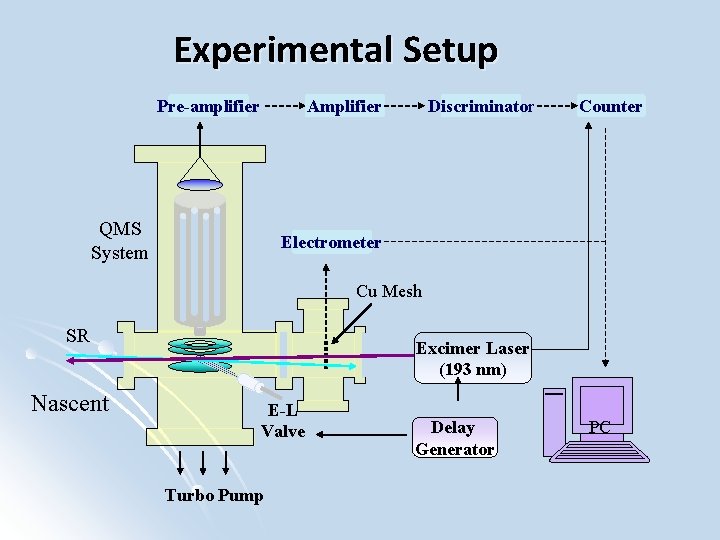
Experimental Setup Pre-amplifier Amplifier QMS System Discriminator Counter Electrometer Cu Mesh SR Nascent Excimer Laser (193 nm) E-L Valve Turbo Pump Delay Generator PC

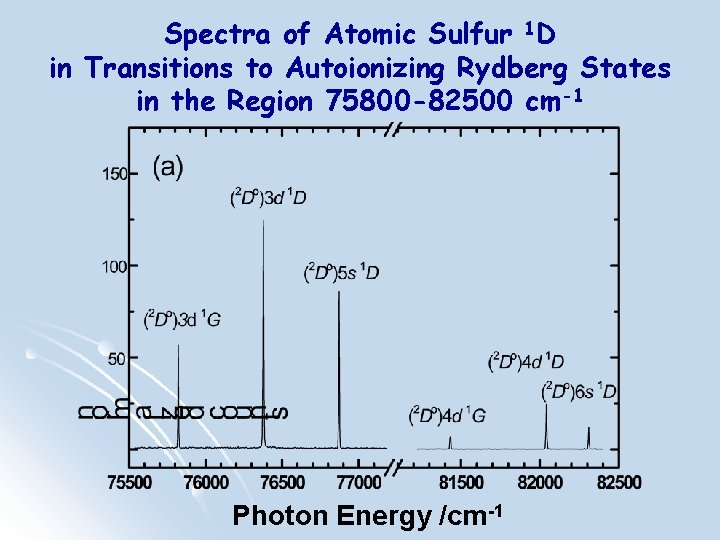
Spectra of Atomic Sulfur 1 D in Transitions to Autoionizing Rydberg States in the Region 75800 -82500 cm-1 Photon Energy /cm-1

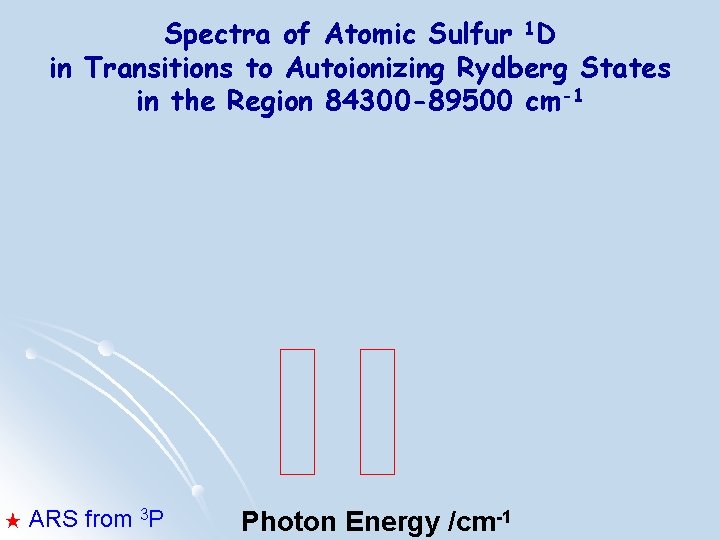
Spectra of Atomic Sulfur 1 D in Transitions to Autoionizing Rydberg States in the Region 84300 -89500 cm-1 ★ ARS from 3 P Photon Energy /cm-1

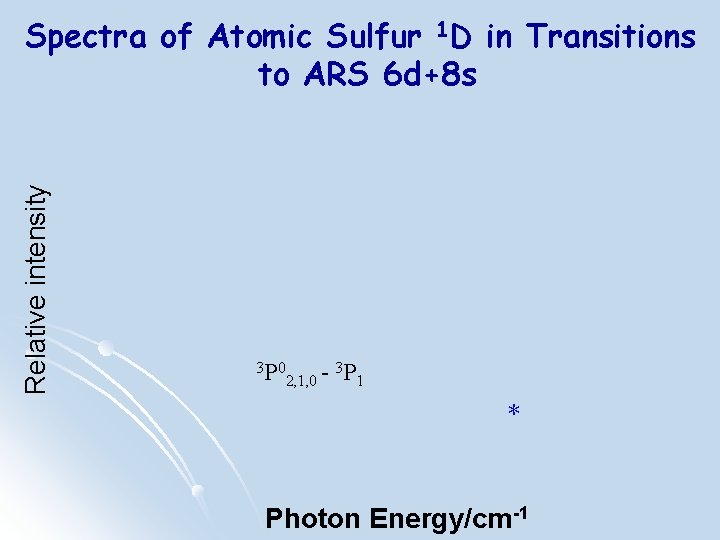
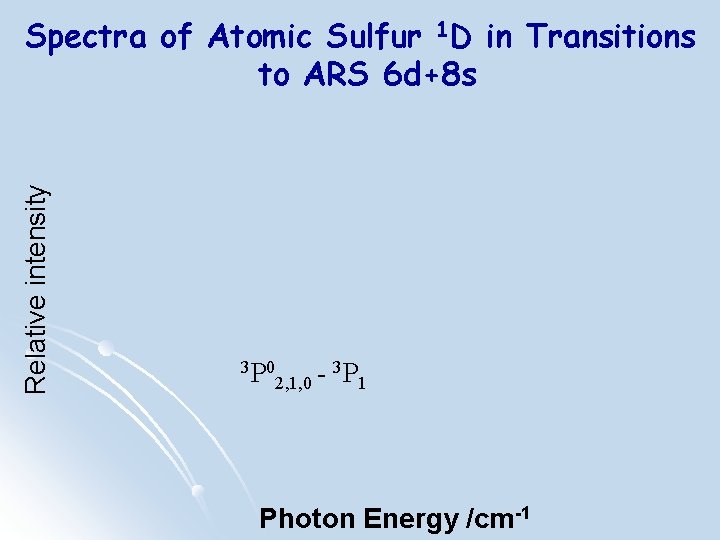
Relative intensity Spectra of Atomic Sulfur 1 D in Transitions to ARS 6 d+8 s 3 P 0 2, 1, 0 - 3 P 1 * Photon Energy/cm-1

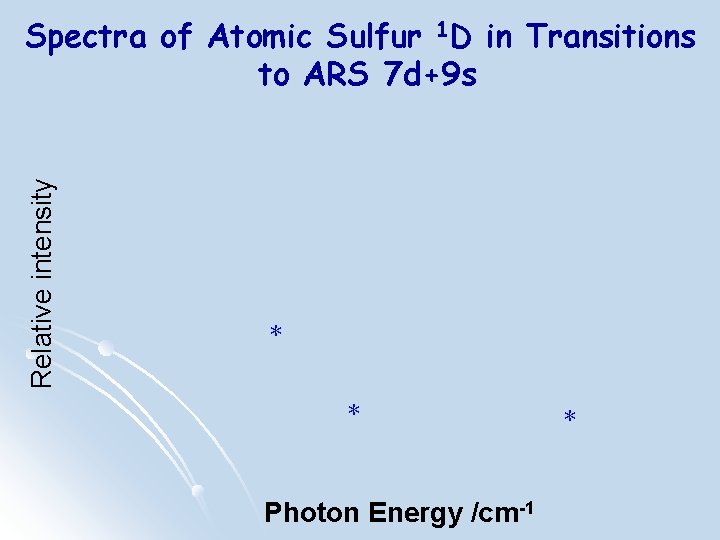
Relative intensity Spectra of Atomic Sulfur 1 D in Transitions to ARS 7 d+9 s * * Photon Energy /cm-1 *

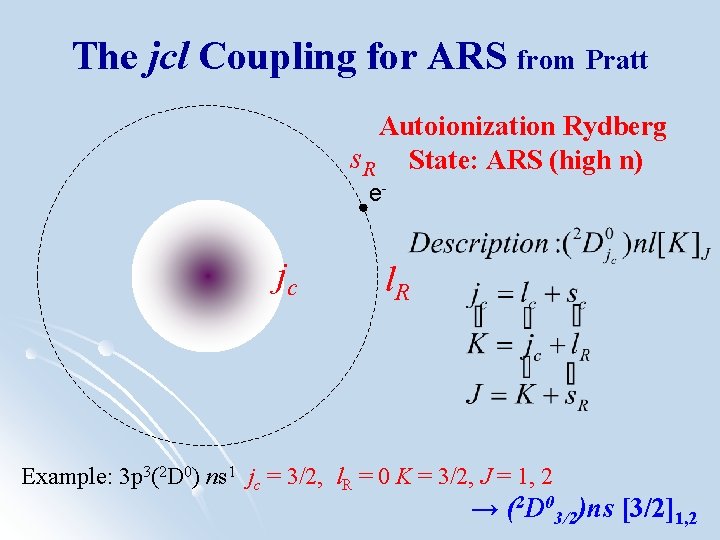
The jcl Coupling for ARS from Pratt Autoionization Rydberg s. R State: ARS (high n) e- jc l. R Example: 3 p 3(2 D 0) ns 1 jc = 3/2, l. R = 0 K = 3/2, J = 1, 2 → (2 D 03/2)ns [3/2]1, 2

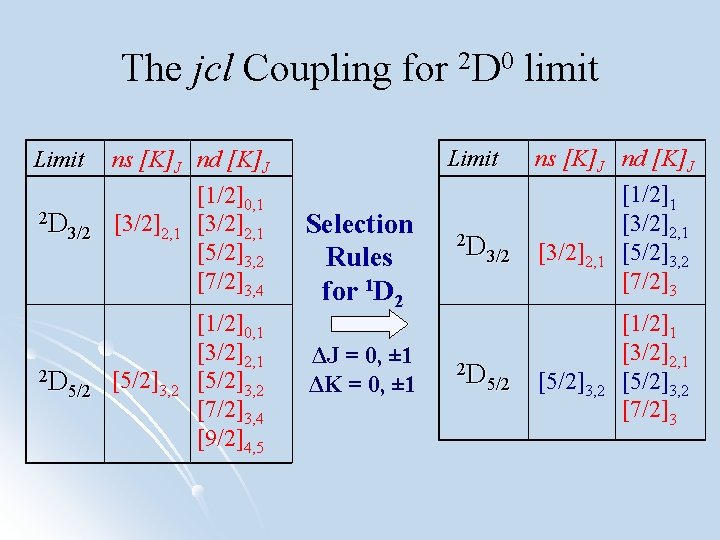
The jcl Coupling for 2 D 0 limit Limit 2 D 3/2 2 D 5/2 Limit ns [K]J nd [K]J [3/2]2, 1 [5/2]3, 2 [1/2]0, 1 [3/2]2, 1 [5/2]3, 2 [7/2]3, 4 [9/2]4, 5 Selection Rules for 1 D 2 ΔJ = 0, ± 1 ΔK = 0, ± 1 2 D 3/2 2 D 5/2 ns [K]J nd [K]J [1/2]1 [3/2]2, 1 [5/2]3, 2 [7/2]3 [5/2]3, 2 [1/2]1 [3/2]2, 1 [5/2]3, 2 [7/2]3

Relative intensity Spectra of Atomic Sulfur 1 D in Transitions to ARS 6 d+8 s 3 P 0 3 P 2, 1, 0 1 Photon Energy /cm-1

Relative intensity Spectra of Atomic Sulfur 1 D in Transitions to ARS 7 d+9 s Photon Energy /cm-1

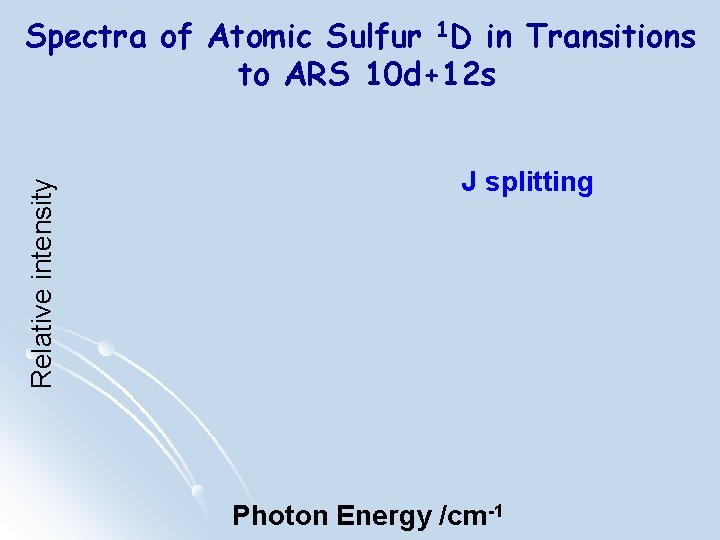
Relative intensity Spectra of Atomic Sulfur 1 D in Transitions to ARS 10 d+12 s J splitting Photon Energy /cm-1

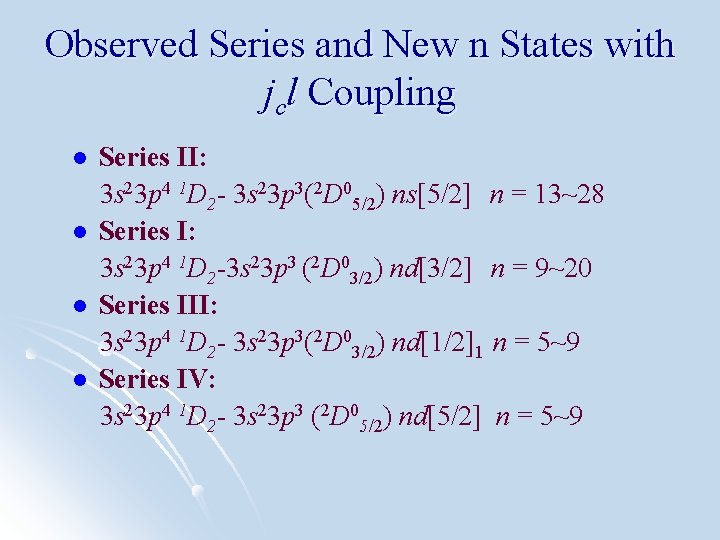
Observed Series and New n States with jcl Coupling l l Series II: 3 s 23 p 4 1 D 2 - 3 s 23 p 3(2 D 05/2) ns[5/2] n = 13~28 Series I: 3 s 23 p 4 1 D 2 -3 s 23 p 3 (2 D 03/2) nd[3/2] n = 9~20 Series III: 3 s 23 p 4 1 D 2 - 3 s 23 p 3(2 D 03/2) nd[1/2]1 n = 5~9 Series IV: 3 s 23 p 4 1 D 2 - 3 s 23 p 3 (2 D 05/2) nd[5/2] n = 5~9
![Low Energy nd Series Er cm1 Elevel cm1 assignment n32 n52 μ32 μ52 Low Energy nd Series Er / cm-1 Elevel /cm-1 assignment n*[3/2] n*[5/2] μ[3/2] μ[5/2]](https://slidetodoc.com/presentation_image_h2/c4aa86799fa658bf05fe938084c5acf5/image-22.jpg)
Low Energy nd Series Er / cm-1 Elevel /cm-1 assignment n*[3/2] n*[5/2] μ[3/2] μ[5/2] Series 1 P 83 387 92 625 3 s 23 p 3(2 Do 3/2) 5 d 1 P 1 4. 355 4. 343 0. 645 (0. 657) 83 515 92 754 3 s 23 p 3(2 Do 5/2) 5 d 1 P 1 4. 404 4. 391 (0. 596 ) 0. 609 85 335 94 573 3 s 23 p 3(2 Do 3/2) 6 d 1 P 1 5. 346 5. 324 0. 654 (0. 676) Series 1 G 75 821 85 059 3 s 23 p 3(2 Do) 3 d 1 G 4 2. 867 2. 863 0. 137 81 435 90 673 3 s 23 p 3(2 Do) 4 d 1 G 4 3. 766 3. 757 0. 239 0. 243
![New Series from jcl Coupling Er cm1 Elevel cm1 assignment n32 n52 μ32 New Series from jcl Coupling Er / cm-1 Elevel /cm-1 assignment n*[3/2] n*[5/2] μ[3/2]](https://slidetodoc.com/presentation_image_h2/c4aa86799fa658bf05fe938084c5acf5/image-23.jpg)
New Series from jcl Coupling Er / cm-1 Elevel /cm-1 assignment n*[3/2] n*[5/2] μ[3/2] μ[5/2] Series V 86 898 96 136 3 s 23 p 3(2 Do 3/2) 7 d[5/2] 6. 945 6. 895 0. 055 (0. 105) 87 434 96 673 3 s 23 p 3(2 Do 3/2) 8 d[5/2] 7. 945 7. 870 0. 055 (0. 130) 87 802 97 040 3 s 23 p 3(2 Do 3/2) 9 d[5/2] 8. 946 8. 840 0. 054 (0. 160) 88 066 97 304 3 s 23 p 3(2 Do 3/2) 10 d[5/2] 9. 956 9. 811 0. 044 (0. 189) 88 260 97 498 3 s 23 p 3(2 Do 3/2) 11 d[5/2] 10. 963 10. 772 0. 037 (0. 228) More theoretical calculations on positions and intensity are requested for low energy 1 G states and high energy K states assignment of J splitting.

Conclusions l We have assigned autoionizing Rydberg series 3 s 23 p 3 (2 D 03/2) nd[3/2] and 3 s 23 p 3(2 D 05/2) ns[5/2] with n extending to 20 and 28 in energy between the first and second ionization limits of sulfur. l New Rydberg series 3 s 23 p 3(2 D 03/2) nd[1/2]1, (2 D 05/2) nd [5/2], and (2 D 03/2) nd[5/2] with n up to 9 for the former two series and 11 for the latter are assigned. l Two new lines are tentatively assigned to 3 s 23 p 3(2 D 0) 3 d 1 G and 4 d 1 G, and another new Rydberg line is assigned to 3 s 23 p 3(2 D 03/2) 6 d 1 P to extend series 1 P to n = 6.

Acknowledgement Advisors: Dr. I-Chia Chen Dr. Yin-Yu Lee Co-workers: Mr. Tzu-Ping Huang Mr. Tzan-Yi Dung Dr. Chao-Yu Chung Mr. Sheng-Jui Lee Group members of Dr. I-Chia Chen Group members of Dr. Shih-Huang Lee Funding: NSRRC, Taiwan National Science Council, Taiwan Dept. of Chemistry, NTHU, Taiwan