Photoelectron Spectroscopy PES Photoelectron Spectroscopy Slide 1 What



- Slides: 12

Photoelectron Spectroscopy (PES)

Photoelectron Spectroscopy Slide 1 • What is PES? o Photoelectron spectroscopy analyzes the kinetic energy distribution of the emitted photoelectrons to study the composition and electronic state of a sample. • PES apparatus iramis. cea. fr

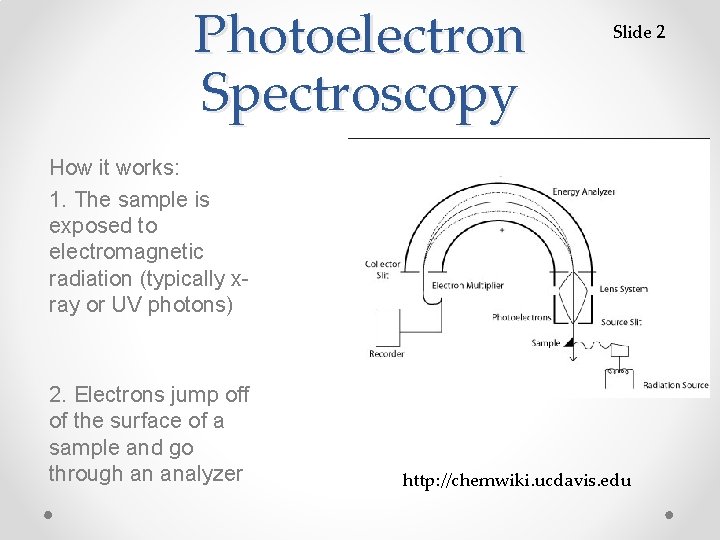
Photoelectron Spectroscopy Slide 2 How it works: 1. The sample is exposed to electromagnetic radiation (typically xray or UV photons) 2. Electrons jump off of the surface of a sample and go through an analyzer http: //chemwiki. ucdavis. edu

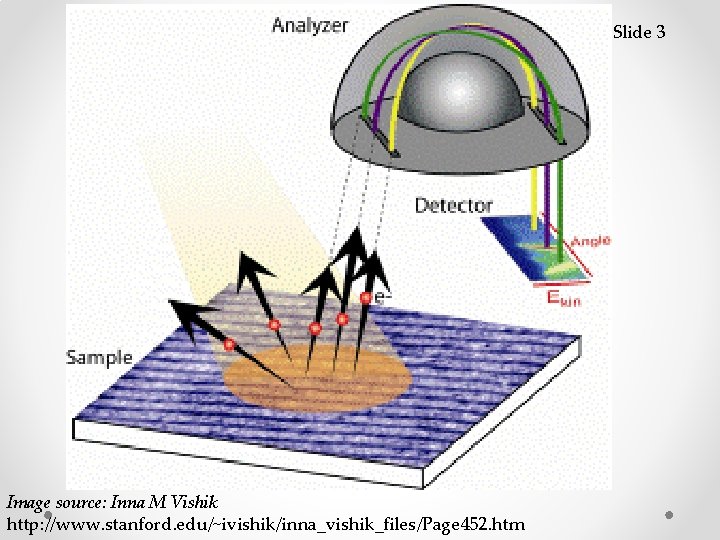
Slide 3 Image source: Inna M Vishik http: //www. stanford. edu/~ivishik/inna_vishik_files/Page 452. htm

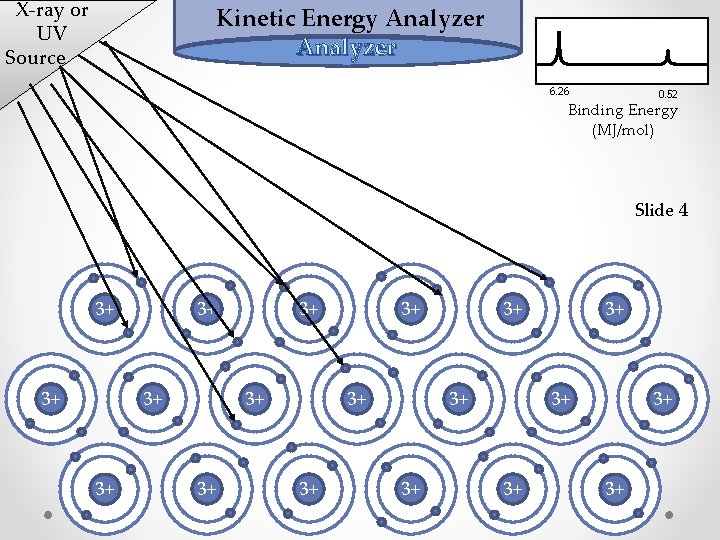
X-ray or UV Source Kinetic Energy Analyzer 6. 26 0. 52 Binding Energy (MJ/mol) Slide 4 3+ 3+ 3+ 3+ 3+

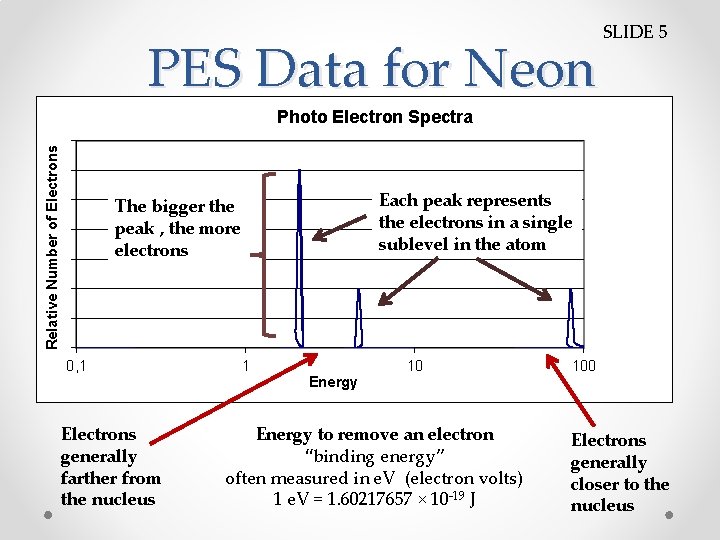
PES Data for Neon SLIDE 5 Relative Number of Electrons Photo Electron Spectra Each peak represents the electrons in a single sublevel in the atom The bigger the peak , the more electrons 0, 1 1 10 100 Energy Electrons generally farther from the nucleus Energy to remove an electron “binding energy” often measured in e. V (electron volts) 1 e. V = 1. 60217657 × 10 -19 J Electrons generally closer to the nucleus

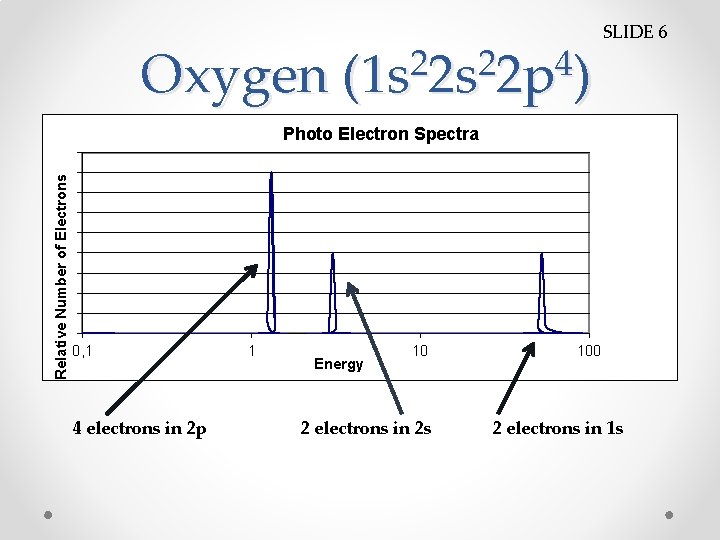
Oxygen 2 2 4 (1 s 2 s 2 p ) SLIDE 6 Relative Number of Electrons Photo Electron Spectra 0, 1 4 electrons in 2 p 1 Energy 10 2 electrons in 2 s 100 2 electrons in 1 s

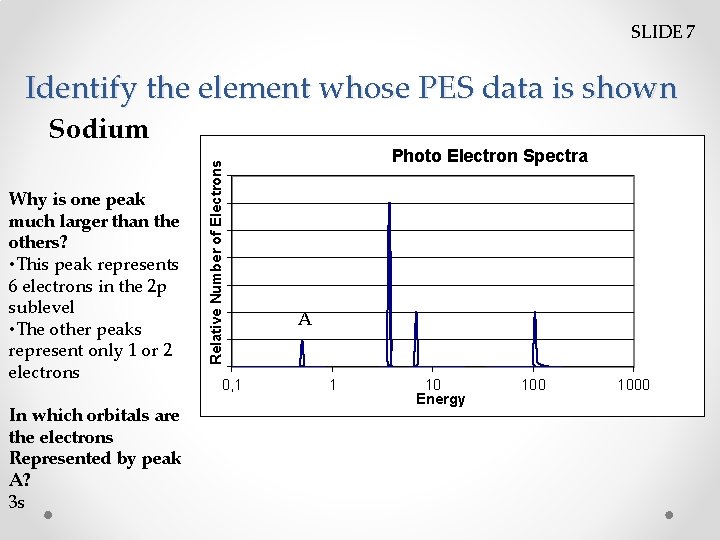
SLIDE 7 Identify the element whose PES data is shown Why is one peak much larger than the others? • This peak represents 6 electrons in the 2 p sublevel • The other peaks represent only 1 or 2 electrons In which orbitals are the electrons Represented by peak A? 3 s Relative Number of Electrons Sodium 0, 1 Photo Electron Spectra A 1 10 Energy 1000

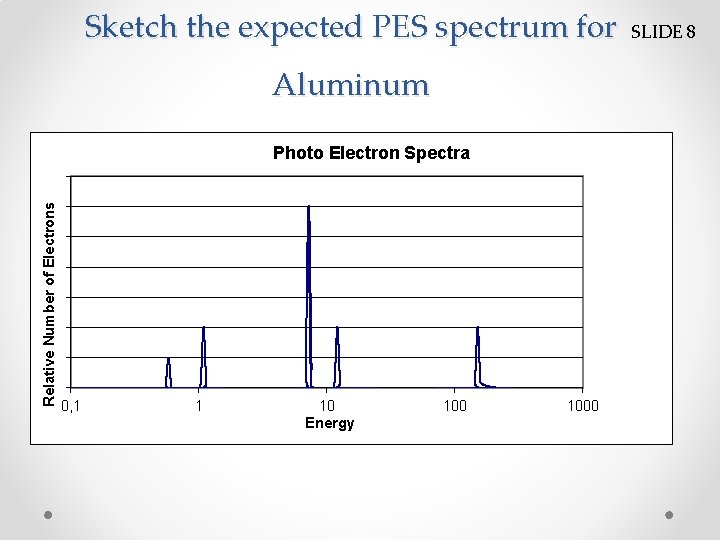
Sketch the expected PES spectrum for Aluminum Relative Number of Electrons Photo Electron Spectra 0, 1 1 10 Energy 1000 SLIDE 8

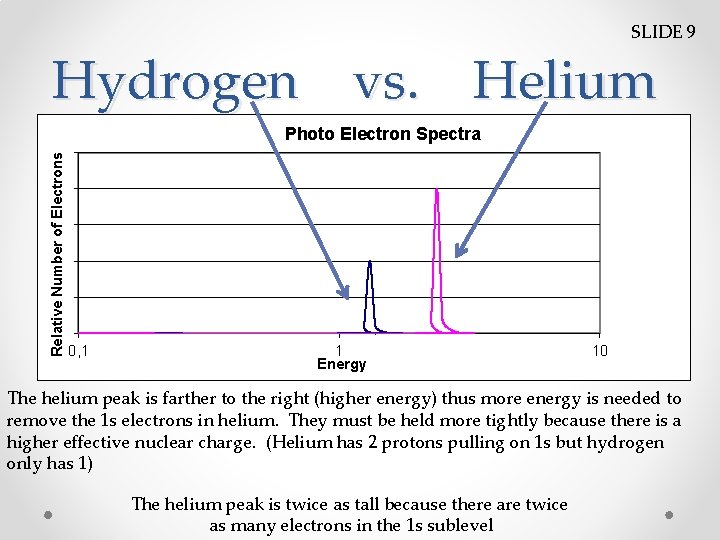
SLIDE 9 Hydrogen vs. Helium Relative Number of Electrons Photo Electron Spectra 0, 1 1 Energy 10 The helium peak is farther to the right (higher energy) thus more energy is needed to remove the 1 s electrons in helium. They must be held more tightly because there is a higher effective nuclear charge. (Helium has 2 protons pulling on 1 s but hydrogen only has 1) The helium peak is twice as tall because there are twice as many electrons in the 1 s sublevel

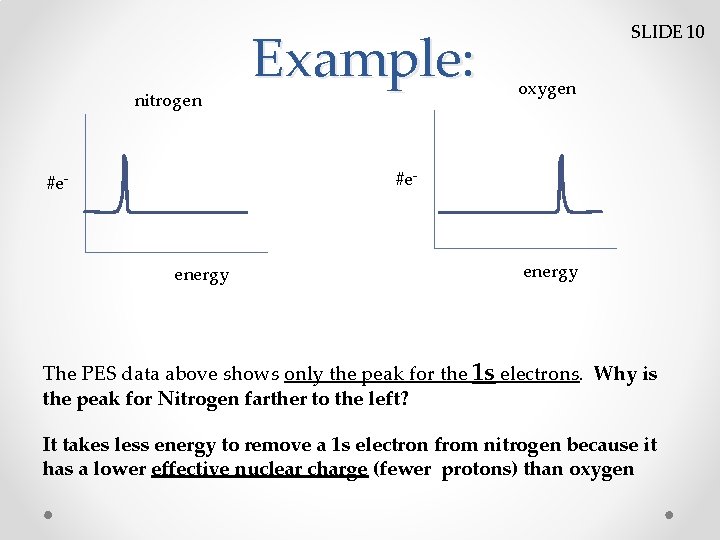
nitrogen Example: SLIDE 10 oxygen #e- energy The PES data above shows only the peak for the 1 s electrons. Why is the peak for Nitrogen farther to the left? It takes less energy to remove a 1 s electron from nitrogen because it has a lower effective nuclear charge (fewer protons) than oxygen

Portions adapted from • http: //teaching. shu. ac. uk/hwb/chemistry/tutorials/molspec/beers 1. ht m • AP 2003 FRQ #5 • Chemistry, Chang, 10 th edition • APSI 2013 OU presentation; J. Beninga • Wikipedia: IR spectroscopy gifs • http: //wwwchem. csustan. edu/Tutorials/images/cychexol. gif • http: //orgchem. colorado. edu/Spectroscopy/irtutor/images/etbenzat. gi f