Photoelectron Spectroscopy PES Key point for today PES






- Slides: 20

Photoelectron Spectroscopy (PES) ***Key point for today: PES provides evidence for the shell model of the atom***

Spectroscopy • Method of analyzing matter using electromagnetic radiation

Energy, Frequency, Wavelength

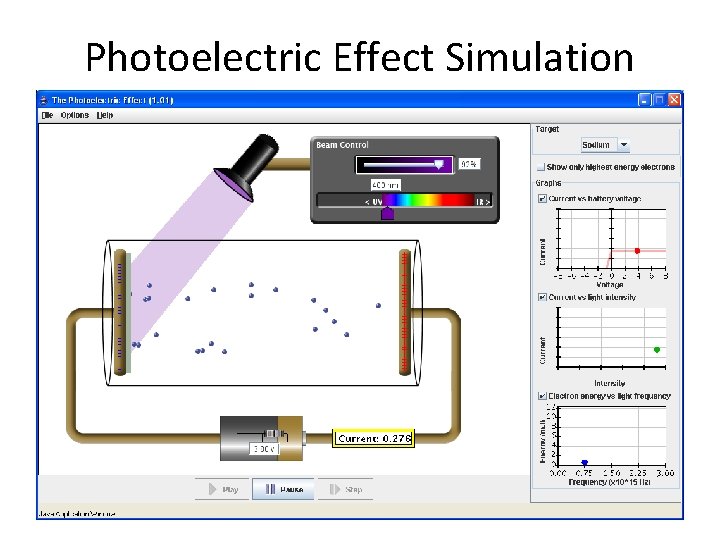
Photoelectric Effect Simulation


• PHET simulation: http: //phet. colorado. edu/en/simulation/phot oelectric • Einstein won the Nobel prize in Physics in 1921 for his explanation of the photoelectric effect (not for his theory of relativity)

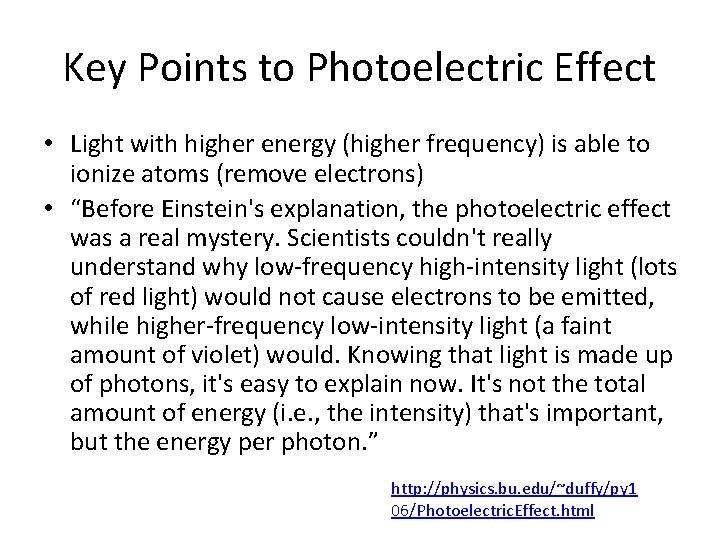
Key Points to Photoelectric Effect • Light with higher energy (higher frequency) is able to ionize atoms (remove electrons) • “Before Einstein's explanation, the photoelectric effect was a real mystery. Scientists couldn't really understand why low-frequency high-intensity light (lots of red light) would not cause electrons to be emitted, while higher-frequency low-intensity light (a faint amount of violet) would. Knowing that light is made up of photons, it's easy to explain now. It's not the total amount of energy (i. e. , the intensity) that's important, but the energy per photon. ” http: //physics. bu. edu/~duffy/py 1 06/Photoelectric. Effect. html

Photoelectron Spectroscopy • PES apparatus: iramis. cea. fr

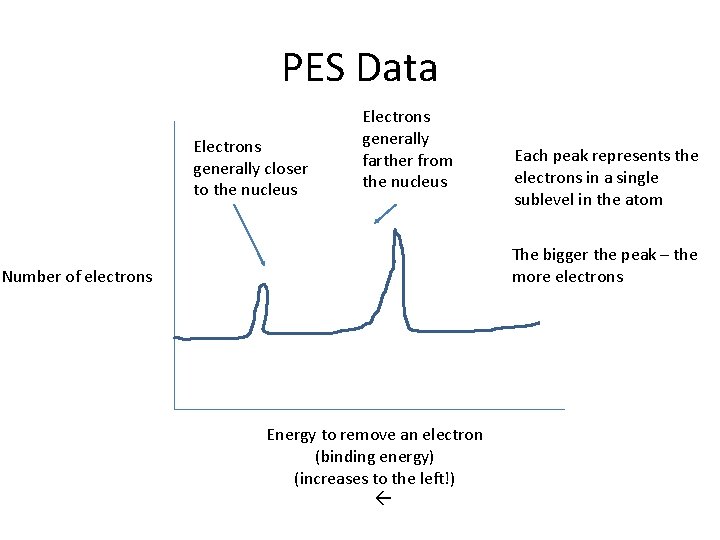
PES Data Electrons generally closer to the nucleus Electrons generally farther from the nucleus Each peak represents the electrons in a single sublevel in the atom The bigger the peak – the more electrons Number of electrons Energy to remove an electron (binding energy) (increases to the left!)

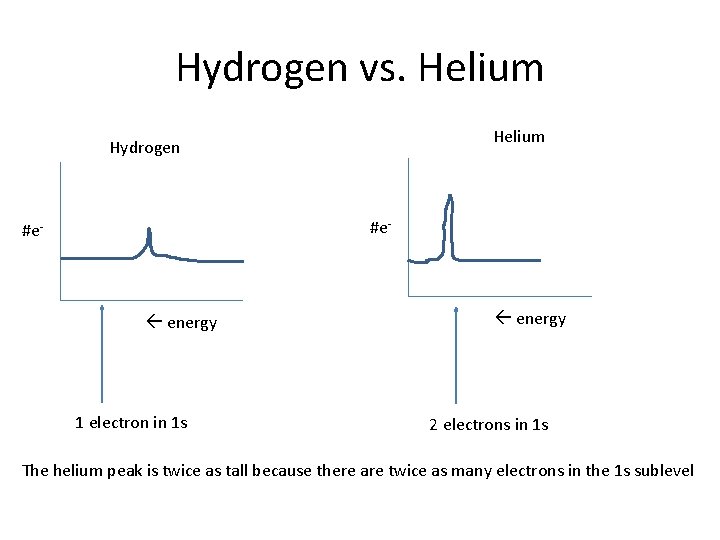
Hydrogen vs. Helium Hydrogen #e- energy 1 electron in 1 s energy 2 electrons in 1 s The helium peak is twice as tall because there are twice as many electrons in the 1 s sublevel

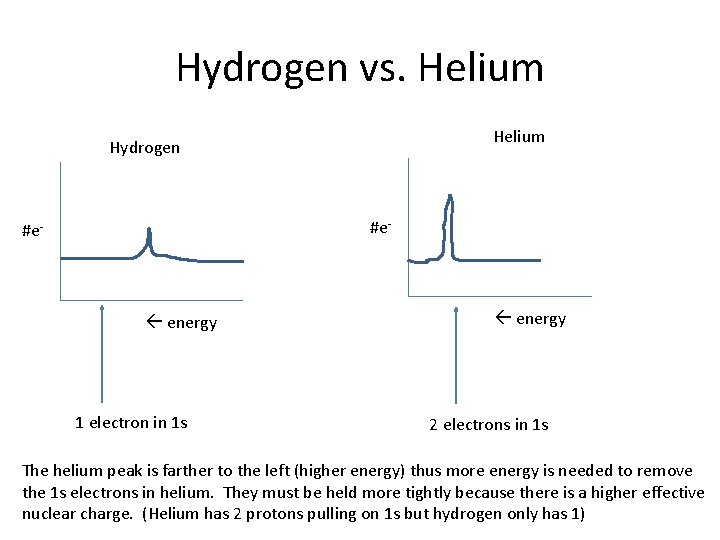
Hydrogen vs. Helium Hydrogen #e- energy 1 electron in 1 s energy 2 electrons in 1 s The helium peak is farther to the left (higher energy) thus more energy is needed to remove the 1 s electrons in helium. They must be held more tightly because there is a higher effective nuclear charge. (Helium has 2 protons pulling on 1 s but hydrogen only has 1)

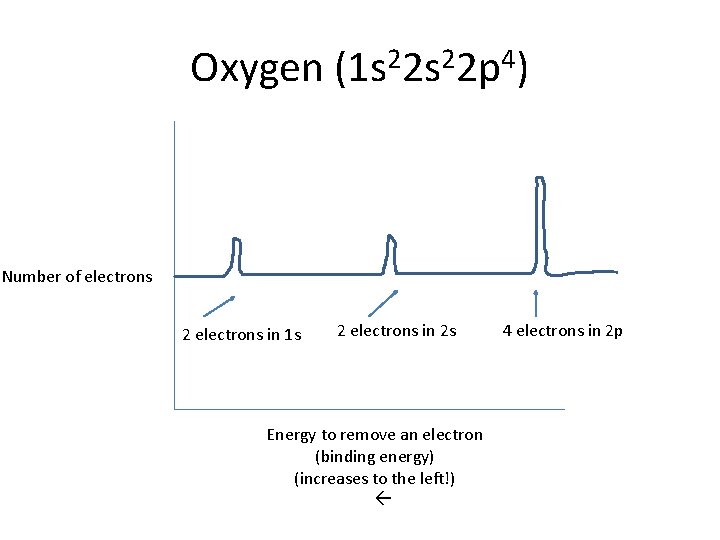
Oxygen (1 s 22 p 4) Number of electrons 2 electrons in 1 s 2 electrons in 2 s Energy to remove an electron (binding energy) (increases to the left!) 4 electrons in 2 p

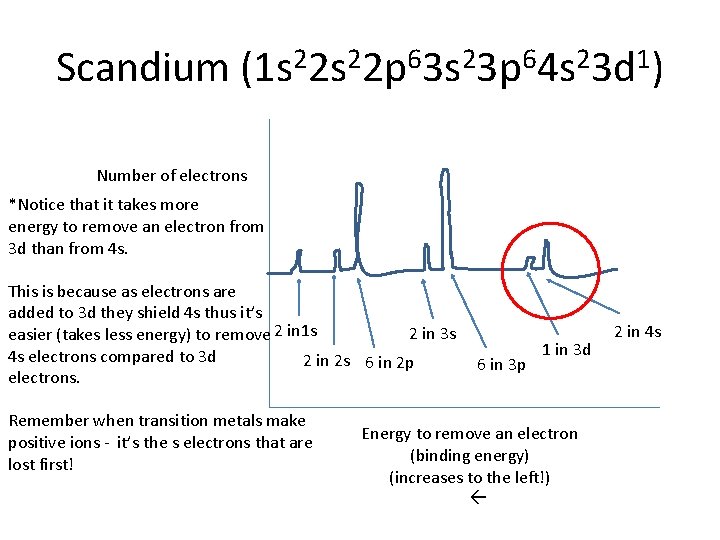
Scandium (1 s 22 p 63 s 23 p 64 s 23 d 1) Number of electrons *Notice that it takes more energy to remove an electron from 3 d than from 4 s. This is because as electrons are added to 3 d they shield 4 s thus it’s 2 in 3 s easier (takes less energy) to remove 2 in 1 s 4 s electrons compared to 3 d 2 in 2 s 6 in 2 p electrons. Remember when transition metals make positive ions - it’s the s electrons that are lost first! 6 in 3 p 1 in 3 d Energy to remove an electron (binding energy) (increases to the left!) 2 in 4 s

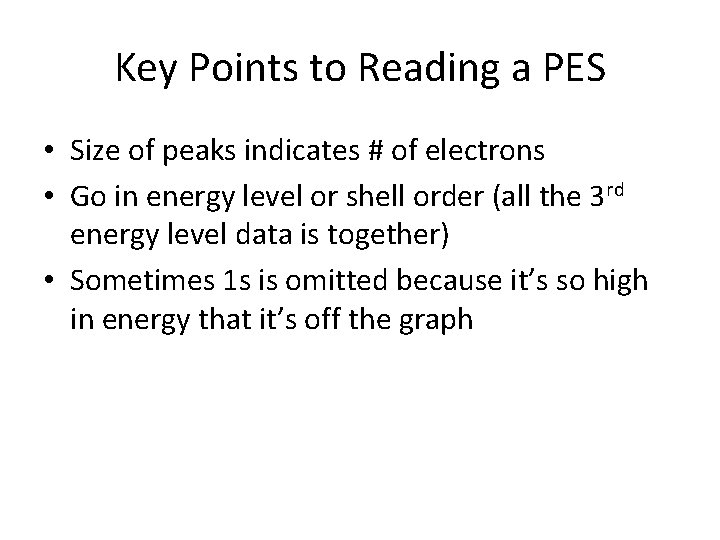
Key Points to Reading a PES • Size of peaks indicates # of electrons • Go in energy level or shell order (all the 3 rd energy level data is together) • Sometimes 1 s is omitted because it’s so high in energy that it’s off the graph

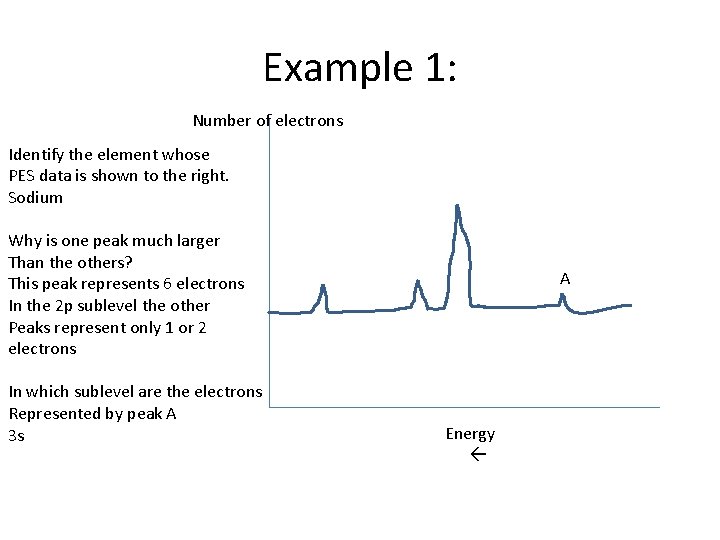
Example 1: Number of electrons Identify the element whose PES data is shown to the right. Sodium Why is one peak much larger Than the others? This peak represents 6 electrons In the 2 p sublevel the other Peaks represent only 1 or 2 electrons In which sublevel are the electrons Represented by peak A 3 s A Energy

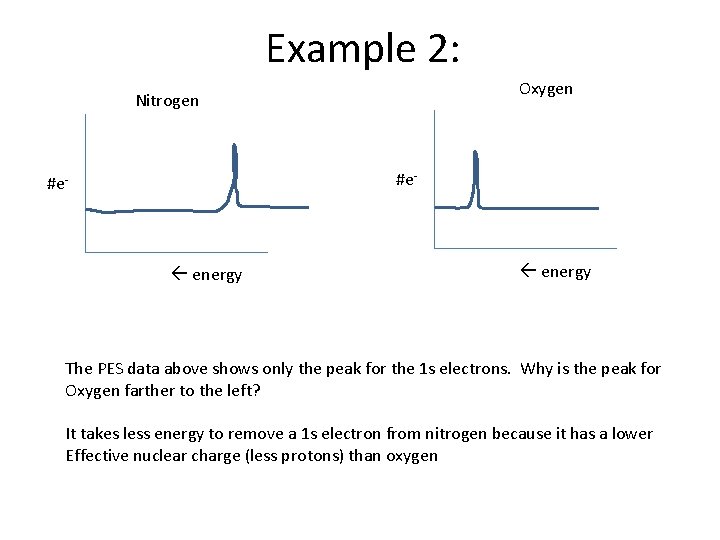
Example 2: Oxygen Nitrogen #e- energy The PES data above shows only the peak for the 1 s electrons. Why is the peak for Oxygen farther to the left? It takes less energy to remove a 1 s electron from nitrogen because it has a lower Effective nuclear charge (less protons) than oxygen

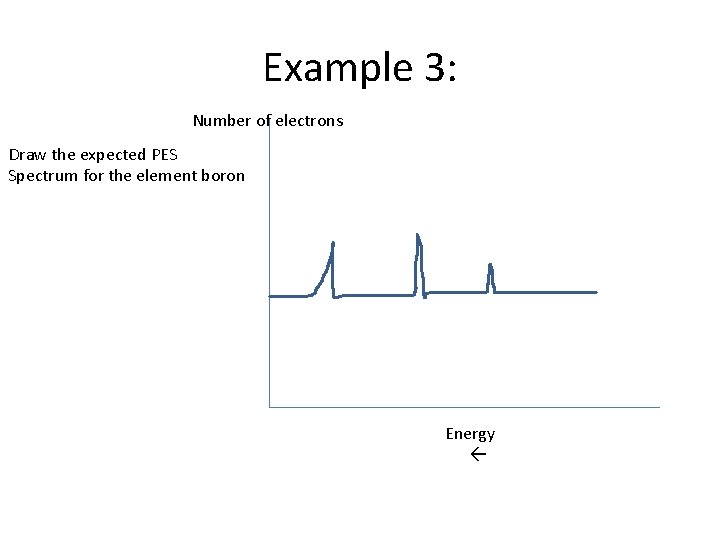
Example 3: Number of electrons Draw the expected PES Spectrum for the element boron Energy

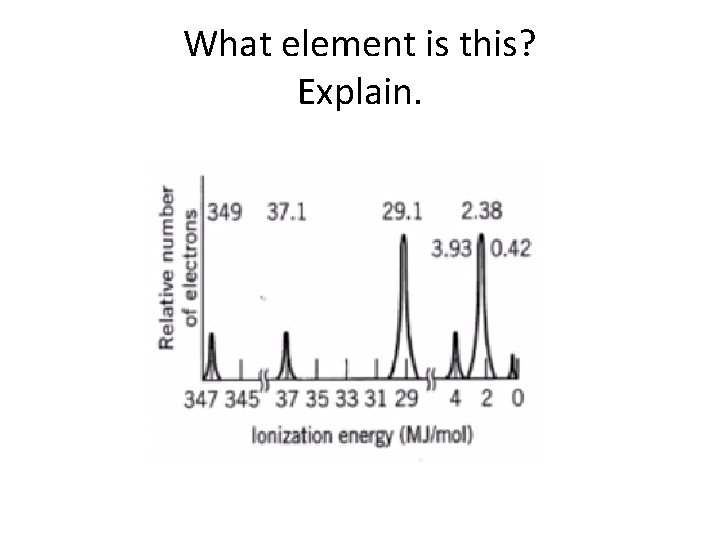
What element is this? Explain.

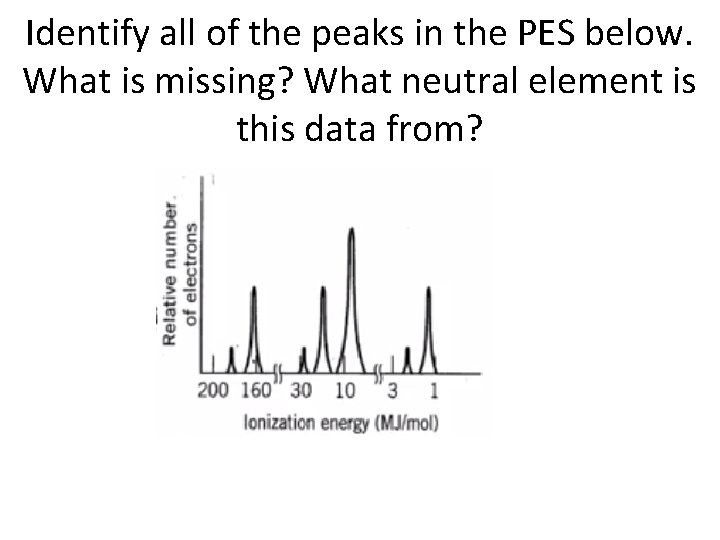
Identify all of the peaks in the PES below. What is missing? What neutral element is this data from?

• Write an electron configuration and draw a PES spectra you might see for aluminum
 Magnesium pes spectrum
Magnesium pes spectrum Spectroscopy ap chem
Spectroscopy ap chem Spectroscopy ap chem
Spectroscopy ap chem Xray photoelectron spectroscopy
Xray photoelectron spectroscopy Beryllium pes
Beryllium pes For today's meeting
For today's meeting Todaysclass
Todaysclass Meeting objective
Meeting objective Fingerprint galton details
Fingerprint galton details Today's lesson or today lesson
Today's lesson or today lesson Example of repitition
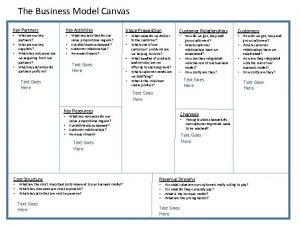
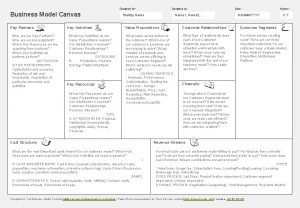
Example of repitition Business canvas example
Business canvas example Key partners
Key partners What was the point of today's lesson
What was the point of today's lesson Fspos
Fspos Typiska drag för en novell
Typiska drag för en novell Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Ekologiskt fotavtryck
Ekologiskt fotavtryck Varför kallas perioden 1918-1939 för mellankrigstiden
Varför kallas perioden 1918-1939 för mellankrigstiden En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Personalliggare bygg undantag
Personalliggare bygg undantag