Photoelectric effect photons Physics 123 3102021 Lecture X








- Slides: 13

Photoelectric effect, photons Physics 123 3/10/2021 Lecture X 1

Concepts • • • Photoelectric effect Work function and stopping potential Electron. Volt (e. V) Photons Blackbody radiation 3/10/2021 Lecture X 2

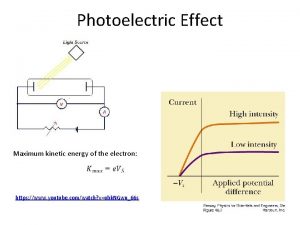
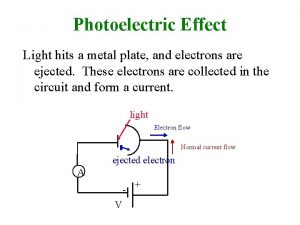
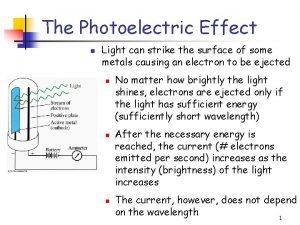
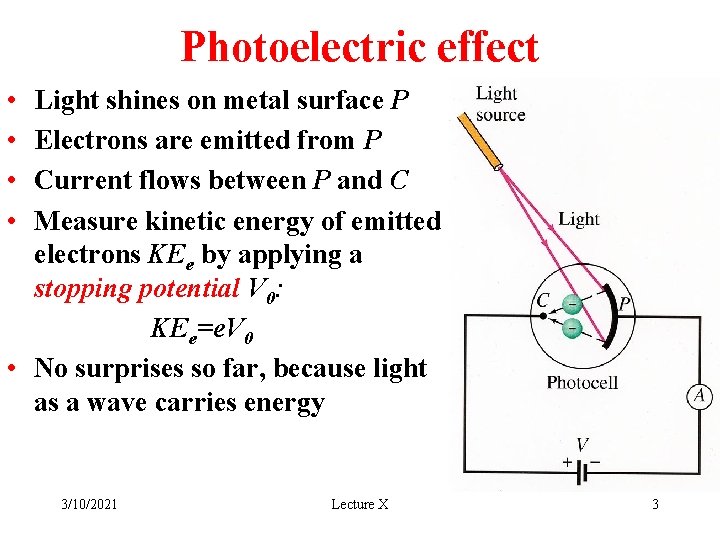
Photoelectric effect • • Light shines on metal surface P Electrons are emitted from P Current flows between P and C Measure kinetic energy of emitted electrons KEe by applying a stopping potential V 0: KEe=e. V 0 • No surprises so far, because light as a wave carries energy 3/10/2021 Lecture X 3

Electronvolt • Energy that one electron gains when being accelerated over 1 V potential difference is called electronvolt e. V: • 1 e. V=1. 6 x 10 -19 C 1 V= 1. 6 x 10 -19 J • Yet another unit to measure energy, • Commonly used in atomic and particle physics. 3/10/2021 Lecture X 4

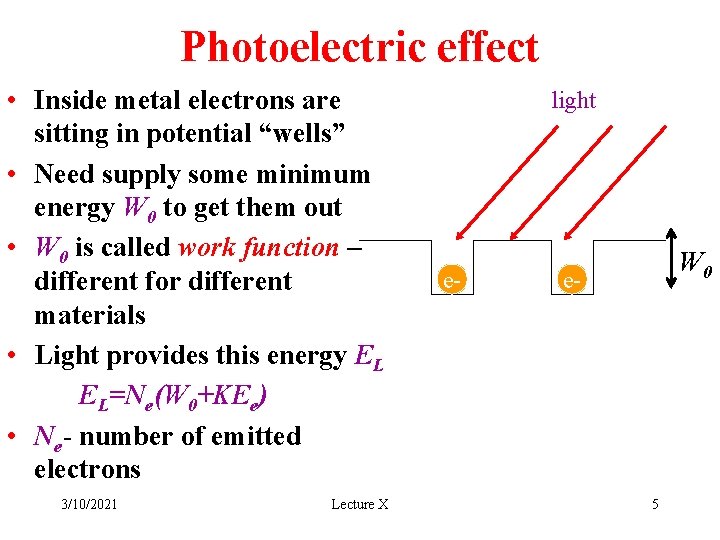
Photoelectric effect • Inside metal electrons are sitting in potential “wells” • Need supply some minimum energy W 0 to get them out • W 0 is called work function – different for different materials • Light provides this energy EL EL=Ne(W 0+KEe) • Ne- number of emitted electrons 3/10/2021 Lecture X light e- W 0 e- 5

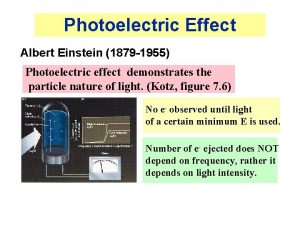
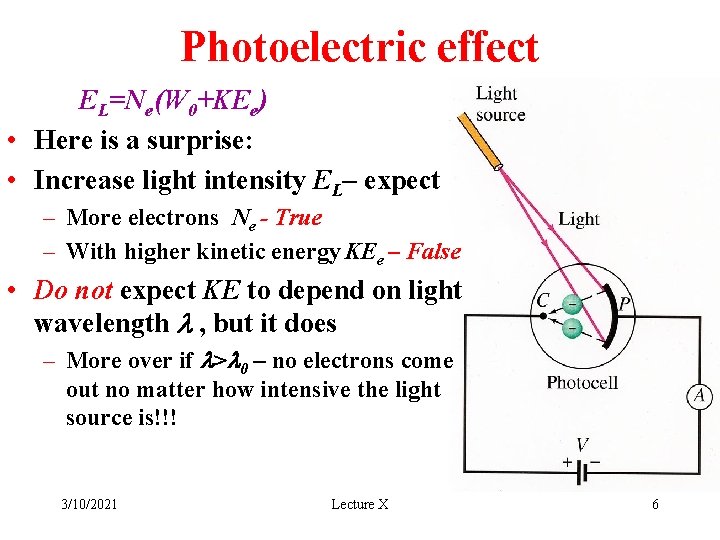
Photoelectric effect EL=Ne(W 0+KEe) • Here is a surprise: • Increase light intensity EL– expect – More electrons Ne - True – With higher kinetic energy KEe – False • Do not expect KE to depend on light wavelength l , but it does – More over if l>l 0 – no electrons come out no matter how intensive the light source is!!! 3/10/2021 Lecture X 6

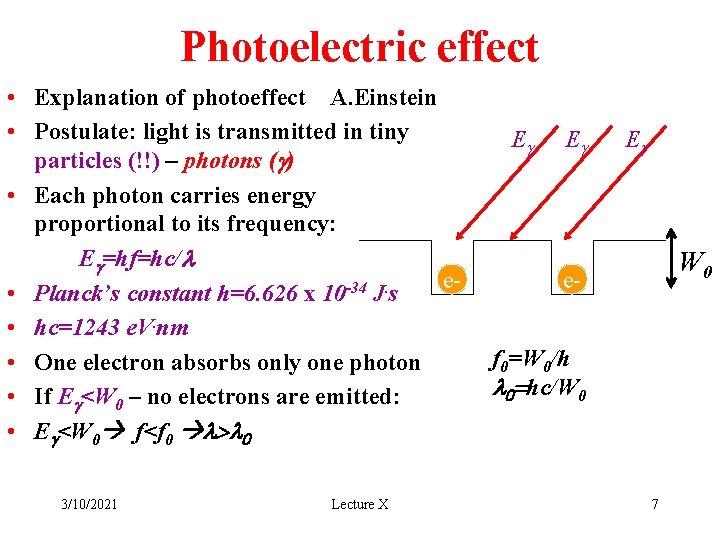
Photoelectric effect • Explanation of photoeffect A. Einstein • Postulate: light is transmitted in tiny particles (!!) – photons (g) • Each photon carries energy proportional to its frequency: Eg=hf=hc/l e-34. • Planck’s constant h=6. 626 x 10 J s • hc=1243 e. V. nm • One electron absorbs only one photon • If Eg<W 0 – no electrons are emitted: • Eg<W 0 f<f 0 l>l 0 3/10/2021 Lecture X Eg Eg Eg W 0 ef 0=W 0/h l 0=hc/W 0 7

Photoelectric effect Eg=hf=hc/l • Photon energy (Eg) is spent to get electron out (W 0) and on electron’s kinetic energy (KEe): Eg= W 0+ KEe e • If stopping potential is applied electron’s kinetic energy KEe is converted into potential energy of the electron e. V 0: Eg= W 0+ e. V 0 3/10/2021 Lecture X Eg Eg Eg KEe W 0 e- 8

Photons – particles of light • Photon= particle of light = quantum of light = gamma(g)-quant • Intensity of electromagnetic wave – sum of energies carried by quanta of light – photons: I=NEg • Each photon carries energy proportional to the frequency of the EM wave: Eg=hf=hc/l 3/10/2021 Lecture X 9

Problem 38 -15 • I=0 when l>l 0=570 nm • Work function W 0 -? • What stopping potential (V 0) must be applied if light of l=400 nm is used? 3/10/2021 Lecture X 10

Photon absorption and emission • Photons can be absorbed by matter – converted into other forms of energy – e. g. kinetic, potential, thermal. At this point photon ceases to exist Final energy=Initial energy+Eg • Photons can be emitted thus reducing the energy of the remaining system. Photon was NOT hiding inside matter waiting to be released, it is created by converting other forms of energy into light (EM-wave) Final energy+Eg=Initial energy • Energy is conserved in either case 3/10/2021 Lecture X 11

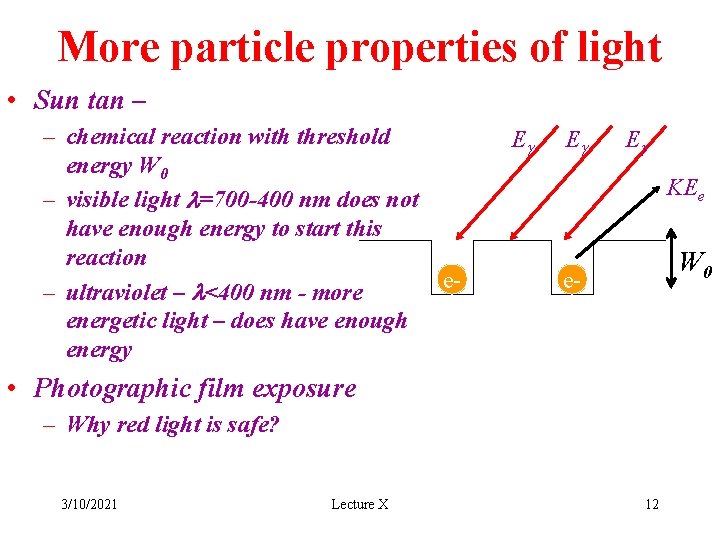
More particle properties of light • Sun tan – – chemical reaction with threshold energy W 0 – visible light l=700 -400 nm does not have enough energy to start this reaction e– ultraviolet – l<400 nm - more energetic light – does have enough energy Eg Eg Eg KEe W 0 e- • Photographic film exposure – Why red light is safe? 3/10/2021 Lecture X 12

Blackbody radiation • Another problem was elegantly solved by introducing “light particles” • Classical explanation of Blackbody (no reflection radiation diverged at low wavelength • Max Planck suggested replacing integral over frequencies with summing a series (photons!) peak lp. T=2. 9 x 10 -3 m. K • Planck did not seek deep meaning behind this seemingly mathematical trick • Fundamental explanation was suggested by Einstein in 1905 – energy is quantized 3/10/2021 Lecture X 13
 Work function formula
Work function formula Photoelectric effect graph explanation
Photoelectric effect graph explanation Compton scattering vs photoelectric effect
Compton scattering vs photoelectric effect Kcvs.ca
Kcvs.ca Photoelectric and compton effect
Photoelectric and compton effect Work function formula
Work function formula Photoelectric effect
Photoelectric effect Photoelectric effect practice problems
Photoelectric effect practice problems Kumar is producing the photoelectric effect by using
Kumar is producing the photoelectric effect by using Multiple choice questions on photoelectric effect
Multiple choice questions on photoelectric effect Hallwachs and lenard's observation on photoelectric effect
Hallwachs and lenard's observation on photoelectric effect Apitlight
Apitlight Rydberg equation
Rydberg equation Photoelectric effect
Photoelectric effect