Phenomics of root system architecture Measuring and analyzing
- Slides: 24

Phenomics of root system architecture: Measuring and analyzing root phenes Larry York and Guillaume Lobet © 2017 American Society of Plant Biologists

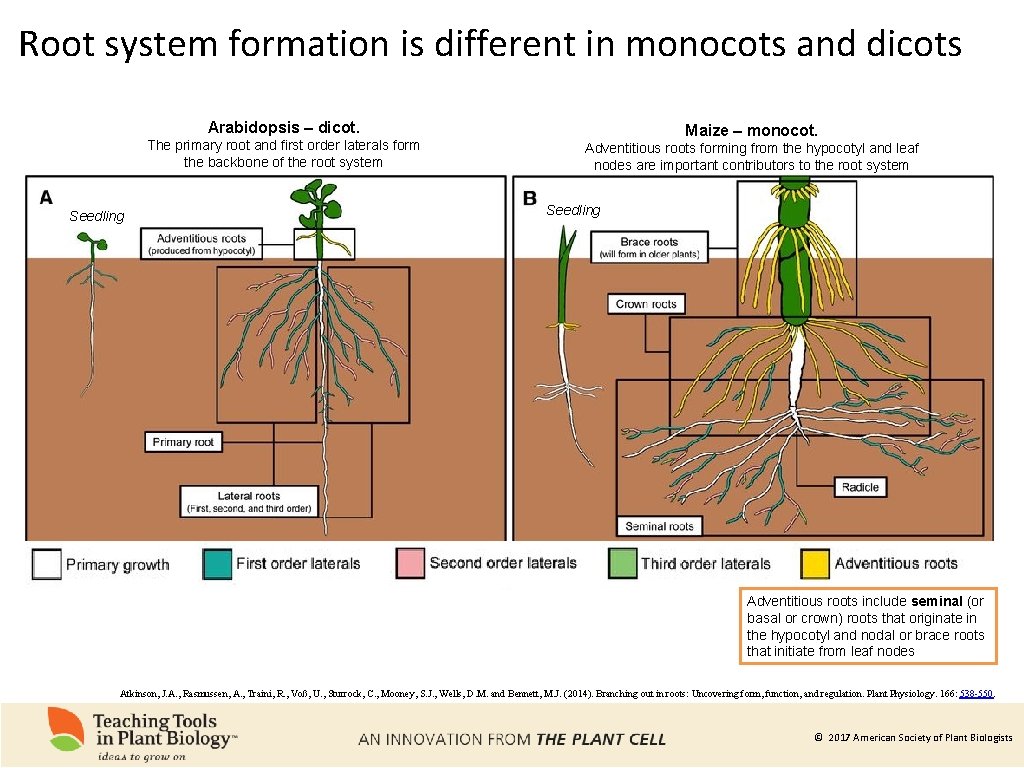
Root system formation is different in monocots and dicots Arabidopsis – dicot. The primary root and first order laterals form the backbone of the root system Seedling Maize – monocot. Adventitious roots forming from the hypocotyl and leaf nodes are important contributors to the root system Seedling Adventitious roots include seminal (or basal or crown) roots that originate in the hypocotyl and nodal or brace roots that initiate from leaf nodes Atkinson, J. A. , Rasmussen, A. , Traini, R. , Voß, U. , Sturrock, C. , Mooney, S. J. , Wells, D. M. and Bennett, M. J. (2014). Branching out in roots: Uncovering form, function, and regulation. Plant Physiology. 166: 538 -550. © 2017 American Society of Plant Biologists

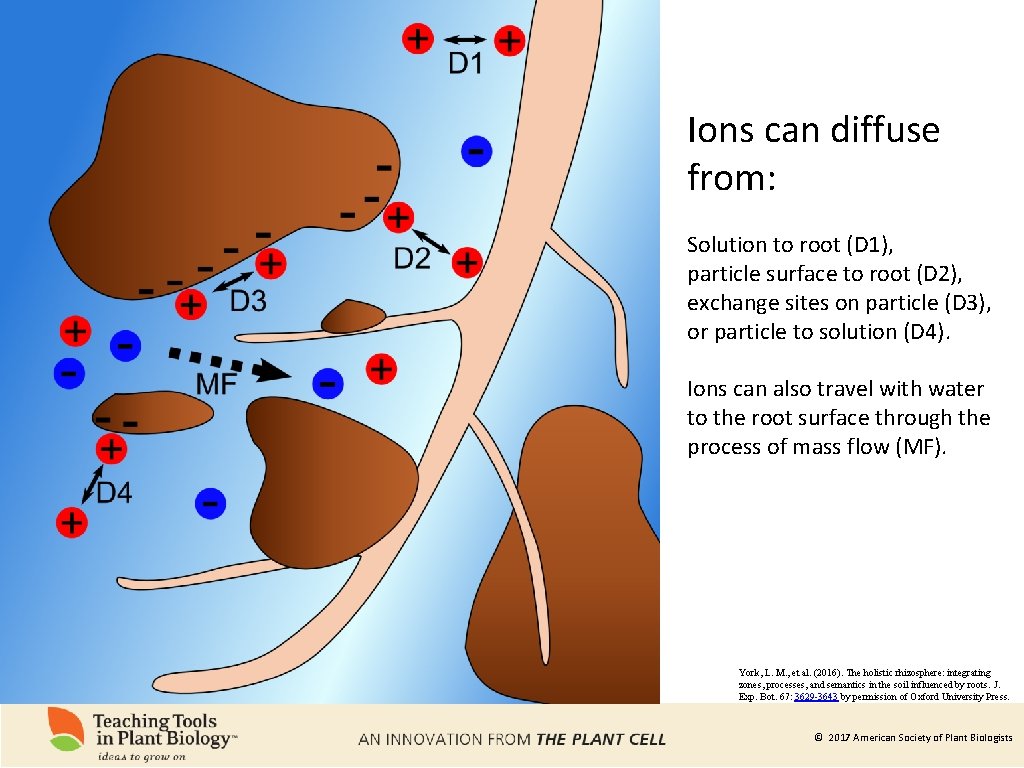
Ions can diffuse from: Solution to root (D 1), particle surface to root (D 2), exchange sites on particle (D 3), or particle to solution (D 4). Ions can also travel with water to the root surface through the process of mass flow (MF). York, L. M. , et al. (2016). The holistic rhizosphere: integrating zones, processes, and semantics in the soil influenced by roots. J. Exp. Bot. 67: 3629 -3643 by permission of Oxford University Press. © 2017 American Society of Plant Biologists

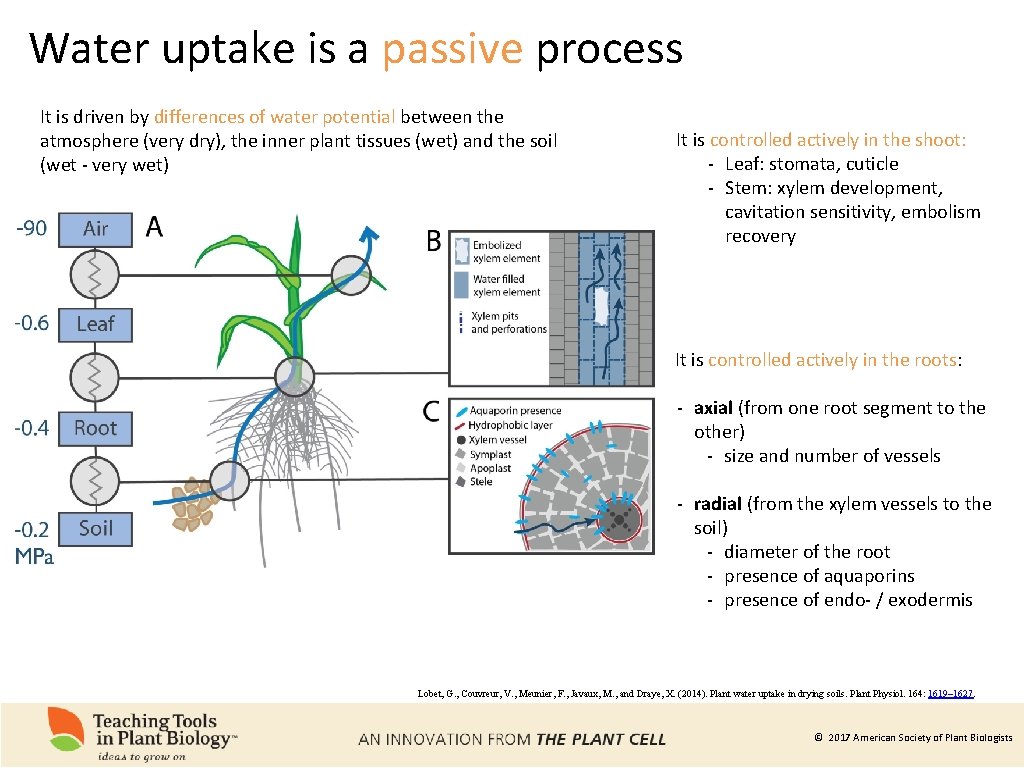
Water uptake is a passive process It is driven by differences of water potential between the atmosphere (very dry), the inner plant tissues (wet) and the soil (wet - very wet) It is controlled actively in the shoot: - Leaf: stomata, cuticle - Stem: xylem development, cavitation sensitivity, embolism recovery It is controlled actively in the roots: - axial (from one root segment to the other) - size and number of vessels - radial (from the xylem vessels to the soil) - diameter of the root - presence of aquaporins - presence of endo- / exodermis Lobet, G. , Couvreur, V. , Meunier, F. , Javaux, M. , and Draye, X. (2014). Plant water uptake in drying soils. Plant Physiol. 164: 1619– 1627. © 2017 American Society of Plant Biologists

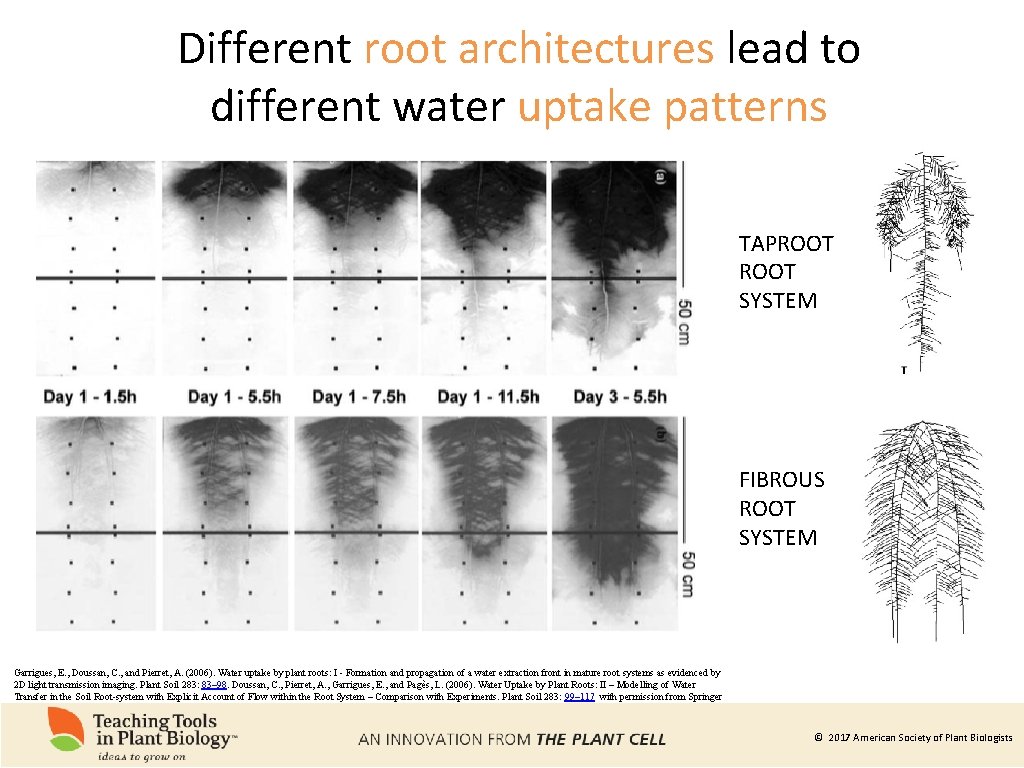
Different root architectures lead to different water uptake patterns TAPROOT SYSTEM FIBROUS ROOT SYSTEM Garrigues, E. , Doussan, C. , and Pierret, A. (2006). Water uptake by plant roots: I - Formation and propagation of a water extraction front in mature root systems as evidenced by 2 D light transmission imaging. Plant Soil 283: 83– 98. Doussan, C. , Pierret, A. , Garrigues, E. , and Pagès, L. (2006). Water Uptake by Plant Roots: II – Modelling of Water Transfer in the Soil Root-system with Explicit Account of Flow within the Root System – Comparison with Experiments. Plant Soil 283: 99– 117 with permission from Springer © 2017 American Society of Plant Biologists

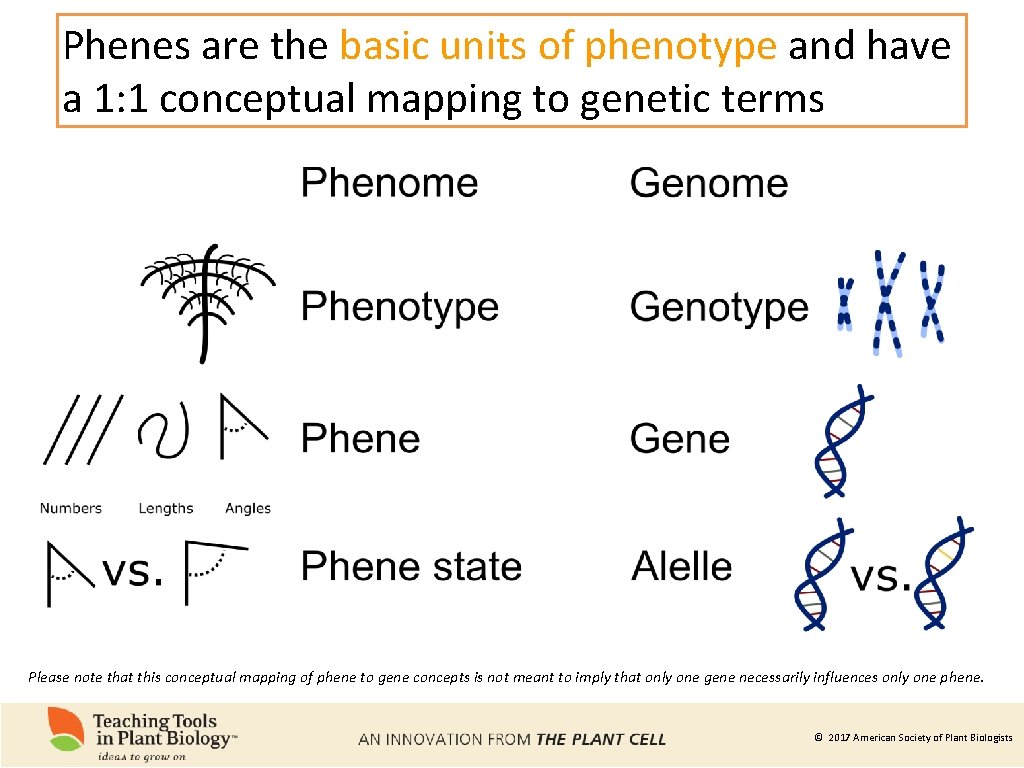
Phenes are the basic units of phenotype and have a 1: 1 conceptual mapping to genetic terms Please note that this conceptual mapping of phene to gene concepts is not meant to imply that only one gene necessarily influences only one phene. © 2017 American Society of Plant Biologists

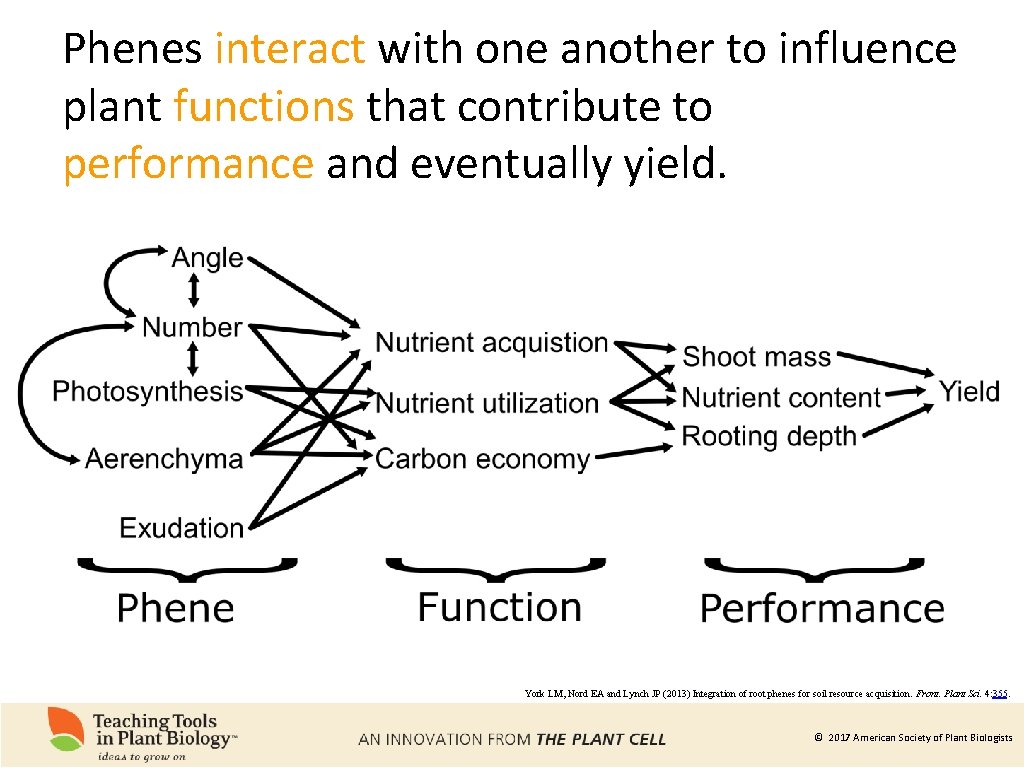
Phenes interact with one another to influence plant functions that contribute to performance and eventually yield. York LM, Nord EA and Lynch JP (2013) Integration of root phenes for soil resource acquisition. Front. Plant Sci. 4: 355. © 2017 American Society of Plant Biologists

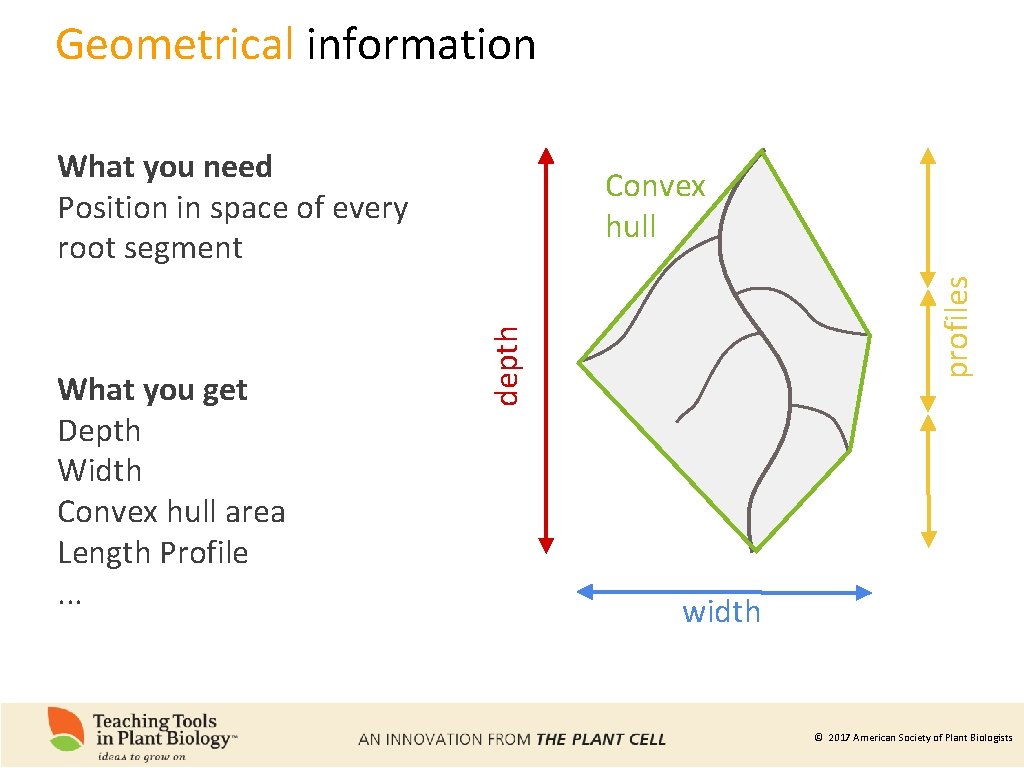
Geometrical information What you need Position in space of every root segment profiles depth What you get Depth Width Convex hull area Length Profile. . . Convex hull width © 2017 American Society of Plant Biologists

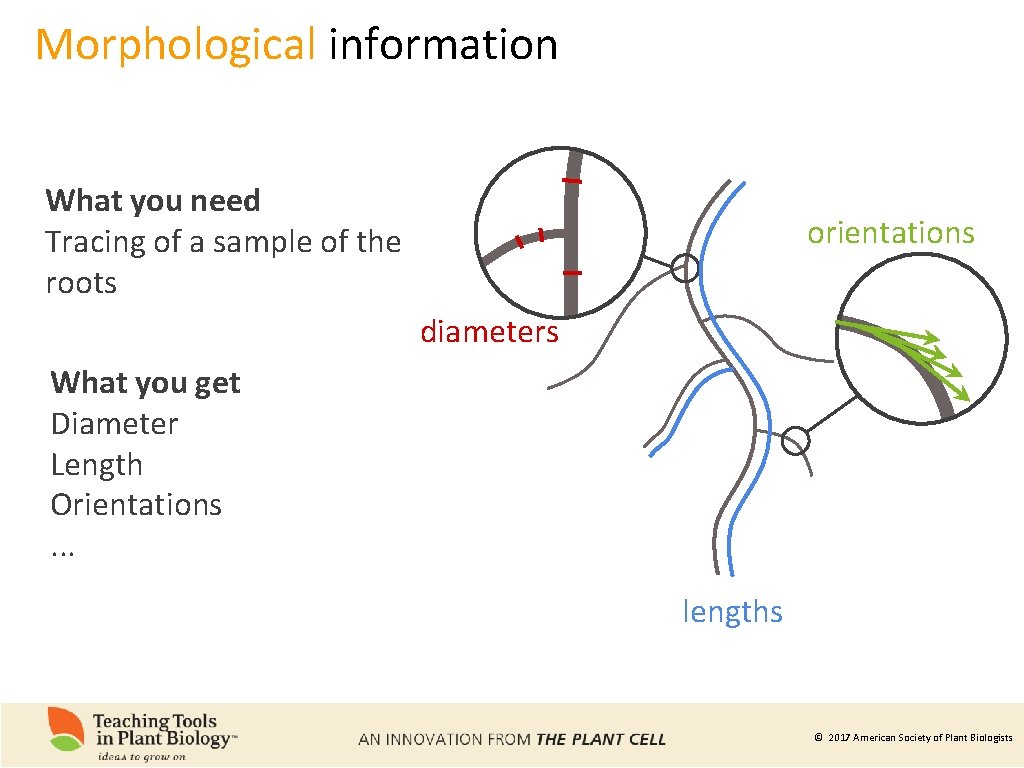
Morphological information What you need Tracing of a sample of the roots orientations diameters What you get Diameter Length Orientations. . . lengths © 2017 American Society of Plant Biologists

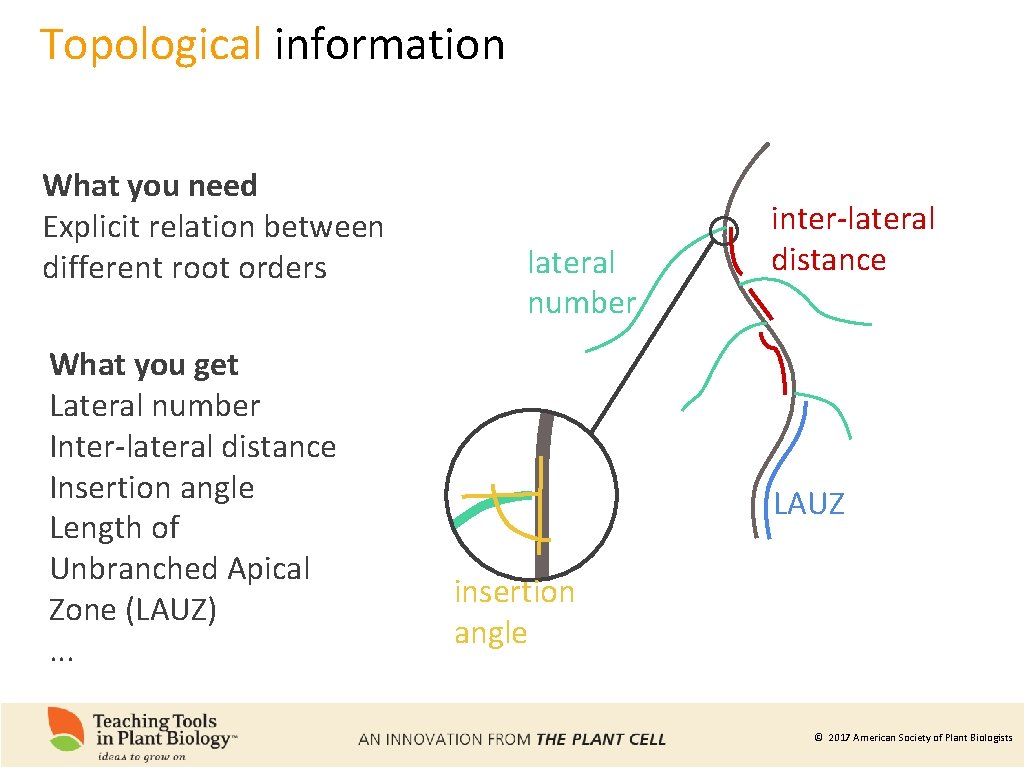
Topological information What you need Explicit relation between different root orders What you get Lateral number Inter-lateral distance Insertion angle Length of Unbranched Apical Zone (LAUZ). . . lateral number inter-lateral distance LAUZ insertion angle © 2017 American Society of Plant Biologists

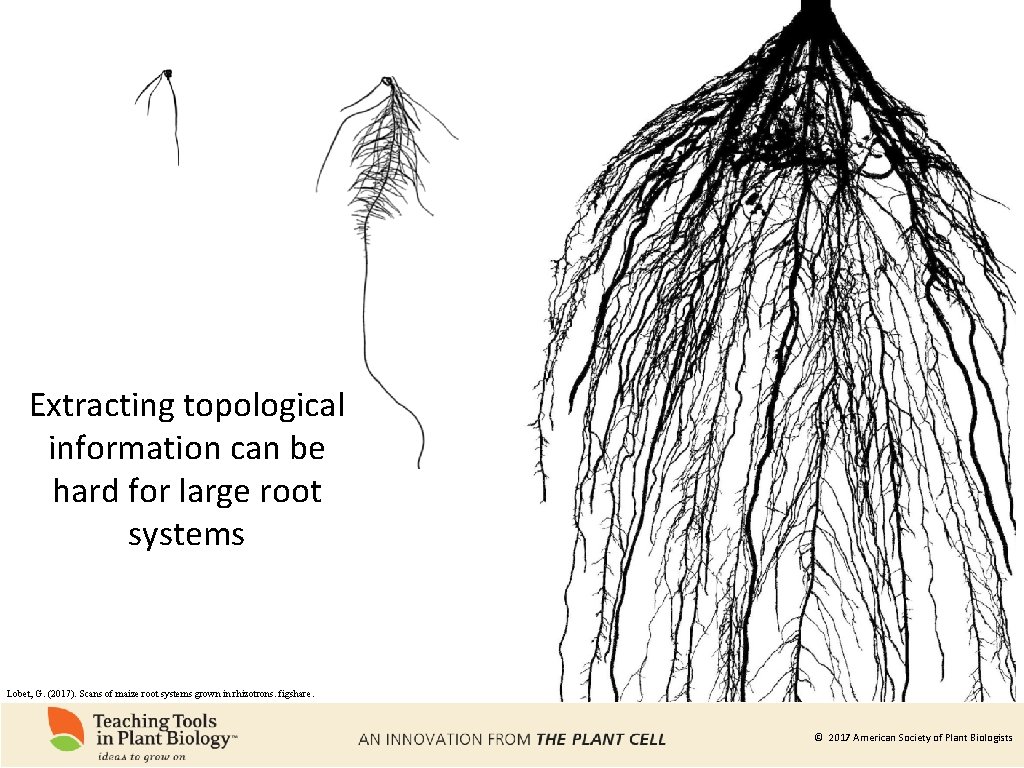
Extracting topological information can be hard for large root systems Lobet, G. (2017). Scans of maize root systems grown in rhizotrons. figshare. © 2017 American Society of Plant Biologists

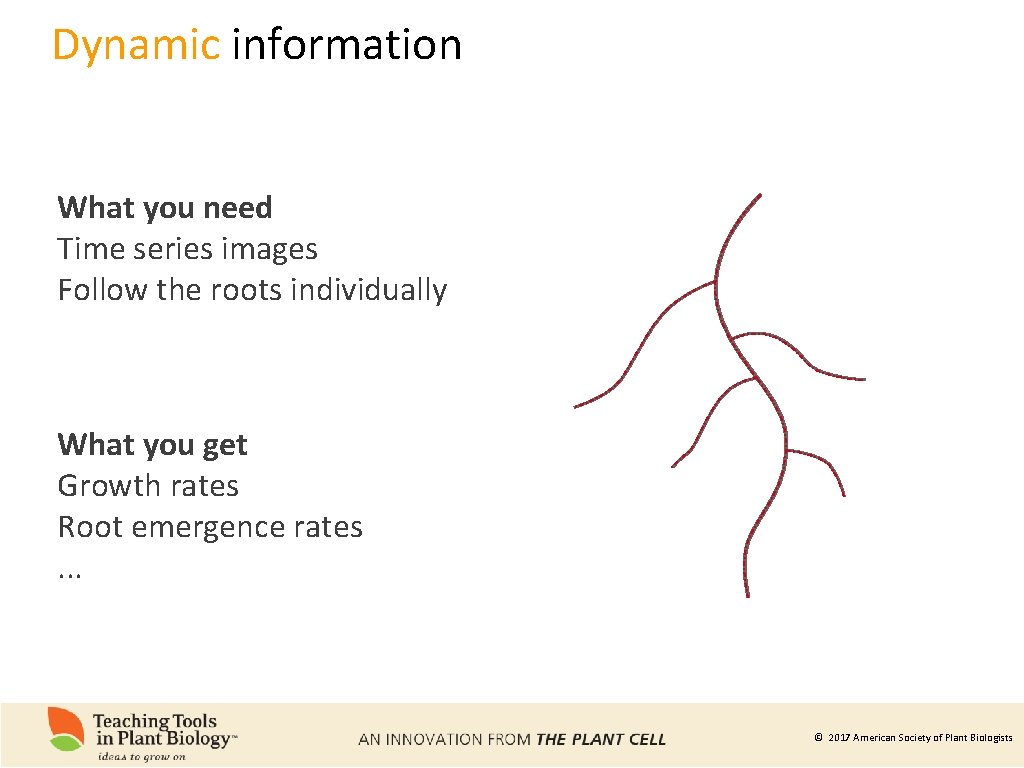
Dynamic information What you need Time series images Follow the roots individually What you get Growth rates Root emergence rates. . . © 2017 American Society of Plant Biologists

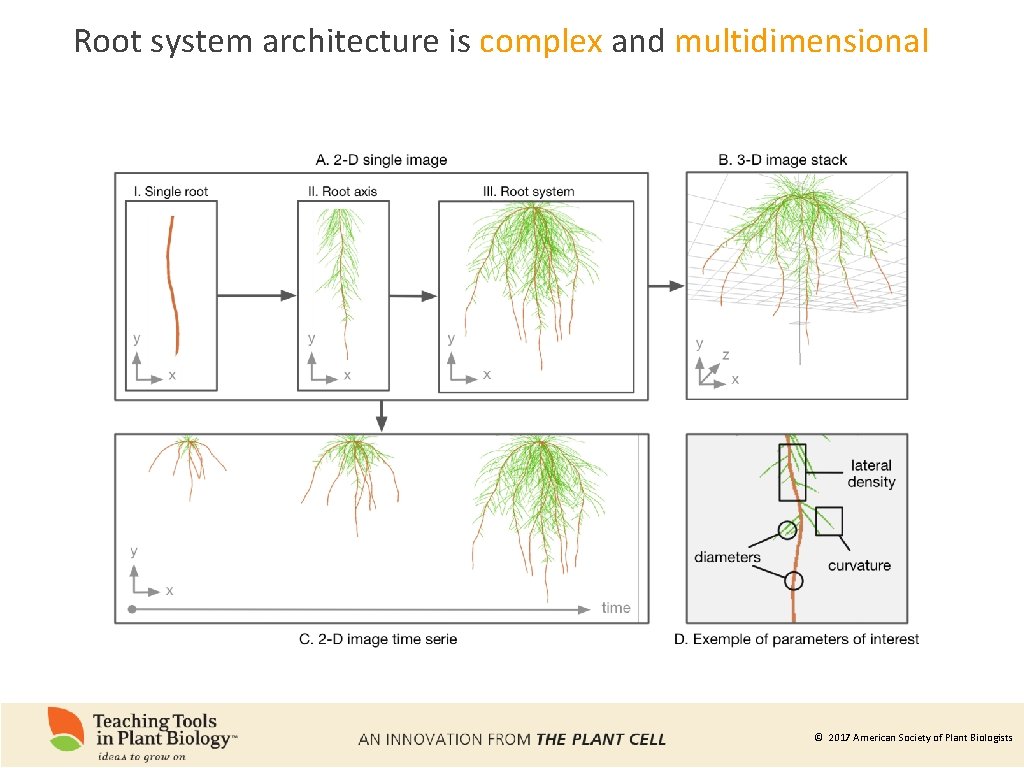
Root system architecture is complex and multidimensional © 2017 American Society of Plant Biologists

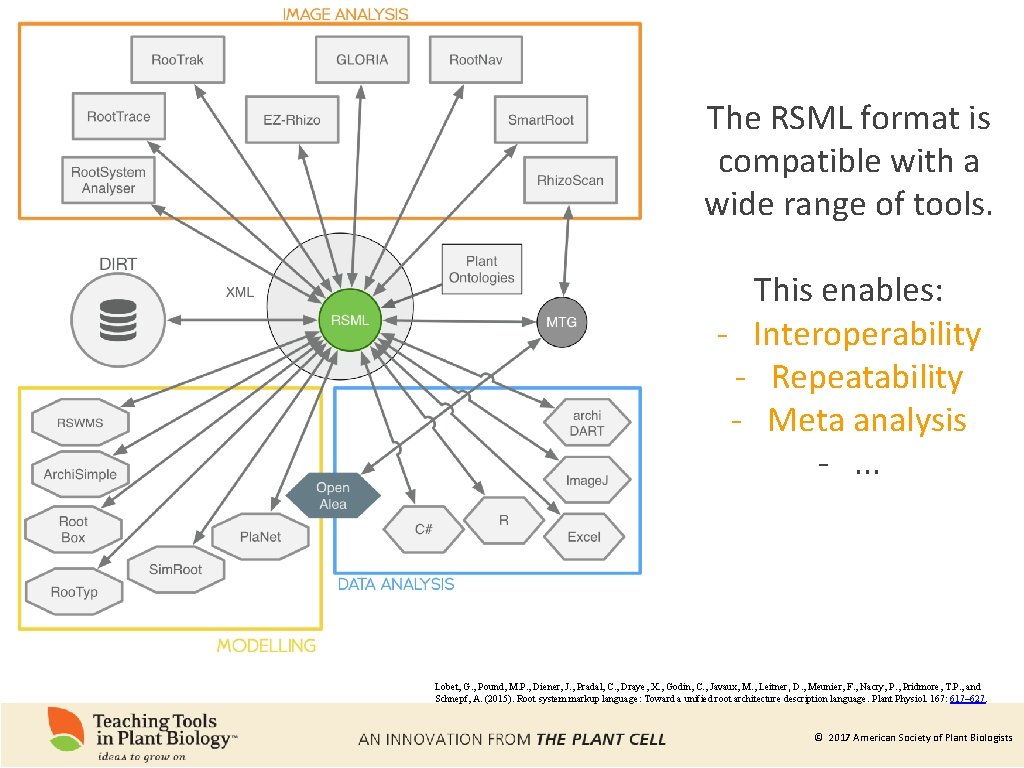
The RSML format is compatible with a wide range of tools. This enables: - Interoperability - Repeatability - Meta analysis -. . . Lobet, G. , Pound, M. P. , Diener, J. , Pradal, C. , Draye, X. , Godin, C. , Javaux, M. , Leitner, D. , Meunier, F. , Nacry, P. , Pridmore, T. P. , and Schnepf, A. (2015). Root system markup language: Toward a unified root architecture description language. Plant Physiol. 167: 617– 627. © 2017 American Society of Plant Biologists

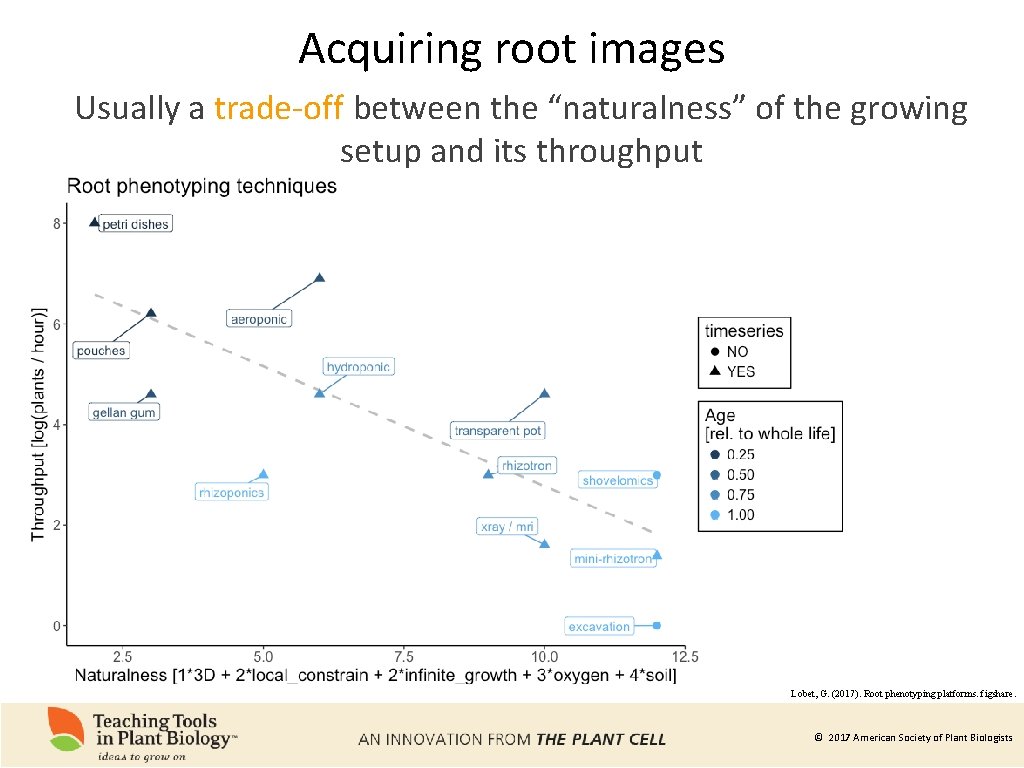
Acquiring root images Usually a trade-off between the “naturalness” of the growing setup and its throughput Lobet, G. (2017). Root phenotyping platforms. figshare. © 2017 American Society of Plant Biologists

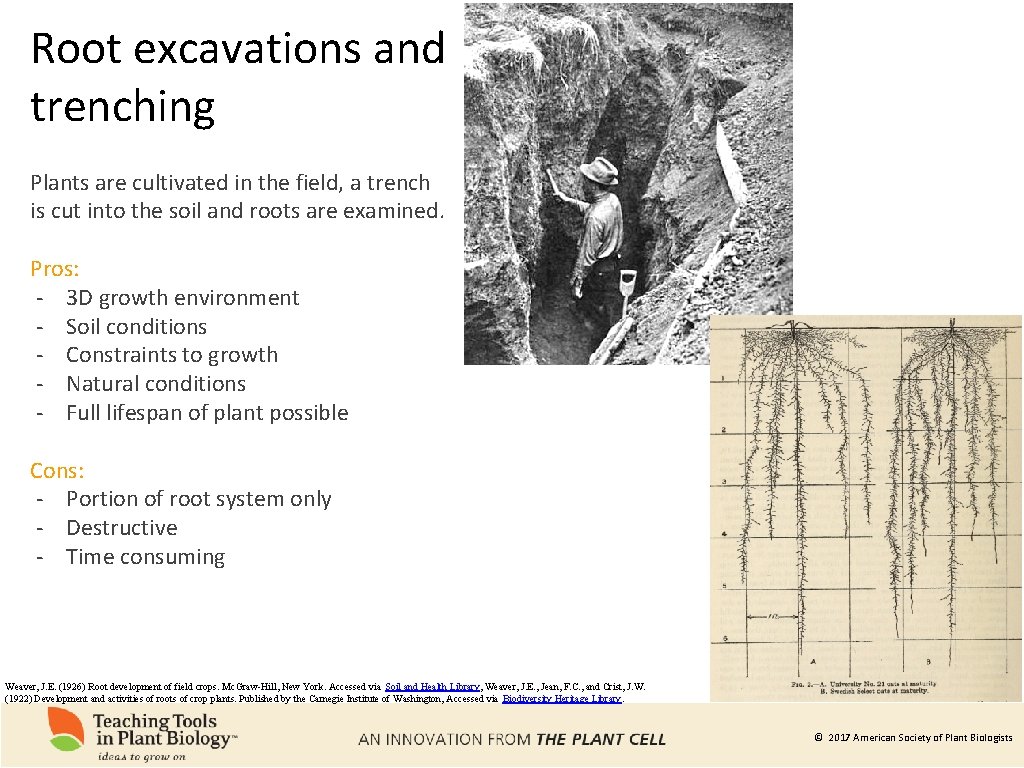
Root excavations and trenching Plants are cultivated in the field, a trench is cut into the soil and roots are examined. Pros: - 3 D growth environment - Soil conditions - Constraints to growth - Natural conditions - Full lifespan of plant possible Cons: - Portion of root system only - Destructive - Time consuming Weaver, J. E. (1926) Root development of field crops. Mc. Graw-Hill, New York. Accessed via Soil and Health Library, Weaver, J. E. , Jean, F. C. , and Crist, J. W. (1922) Development and activities of roots of crop plants. Published by the Carnegie Institute of Washington, Accessed via Biodiversity Heritage Library. © 2017 American Society of Plant Biologists

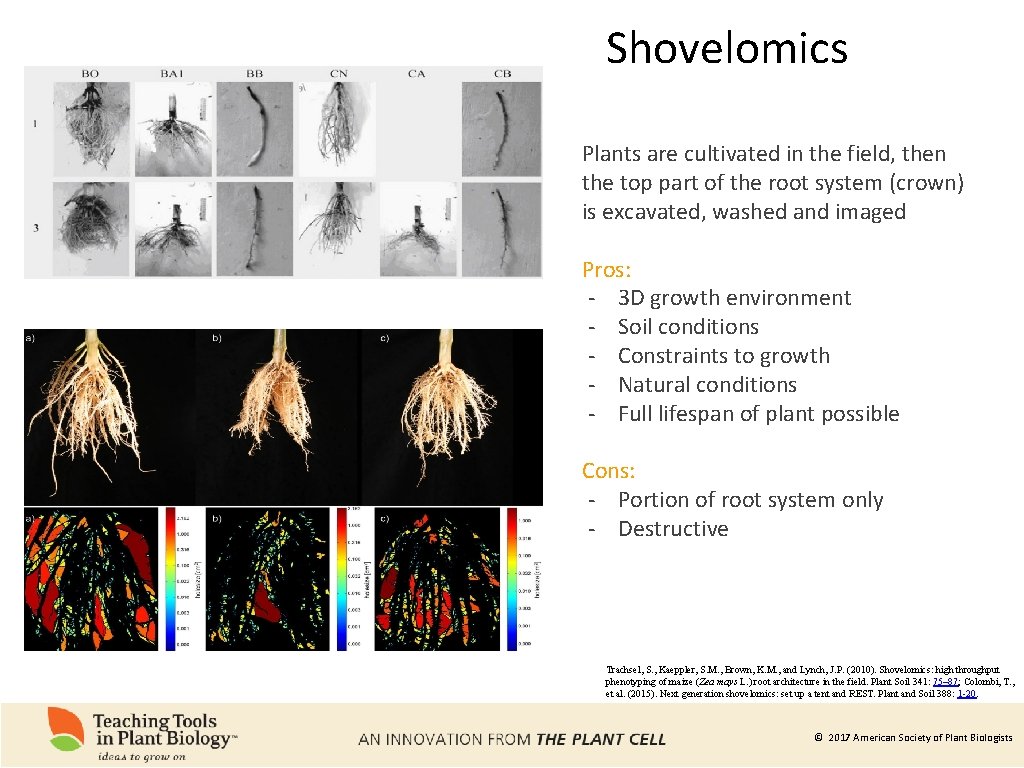
Shovelomics Plants are cultivated in the field, then the top part of the root system (crown) is excavated, washed and imaged Pros: - 3 D growth environment - Soil conditions - Constraints to growth - Natural conditions - Full lifespan of plant possible Cons: - Portion of root system only - Destructive Trachsel, S. , Kaeppler, S. M. , Brown, K. M. , and Lynch, J. P. (2010). Shovelomics: high throughput phenotyping of maize (Zea mays L. ) root architecture in the field. Plant Soil 341: 75– 87; Colombi, T. , et al. (2015). Next generation shovelomics: set up a tent and REST. Plant and Soil 388: 1 -20. © 2017 American Society of Plant Biologists

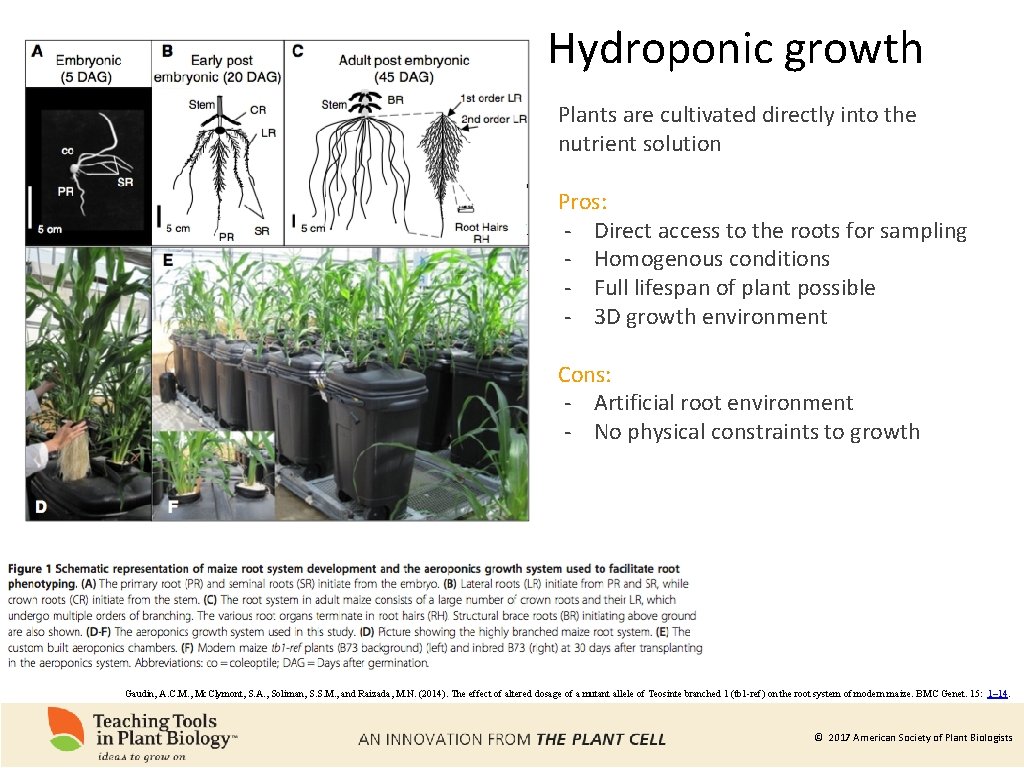
Hydroponic growth Plants are cultivated directly into the nutrient solution Pros: - Direct access to the roots for sampling - Homogenous conditions - Full lifespan of plant possible - 3 D growth environment Cons: - Artificial root environment - No physical constraints to growth Gaudin, A. C. M. , Mc. Clymont, S. A. , Soliman, S. S. M. , and Raizada, M. N. (2014). The effect of altered dosage of a mutant allele of Teosinte branched 1 (tb 1 -ref) on the root system of modern maize. BMC Genet. 15: 1– 14. © 2017 American Society of Plant Biologists

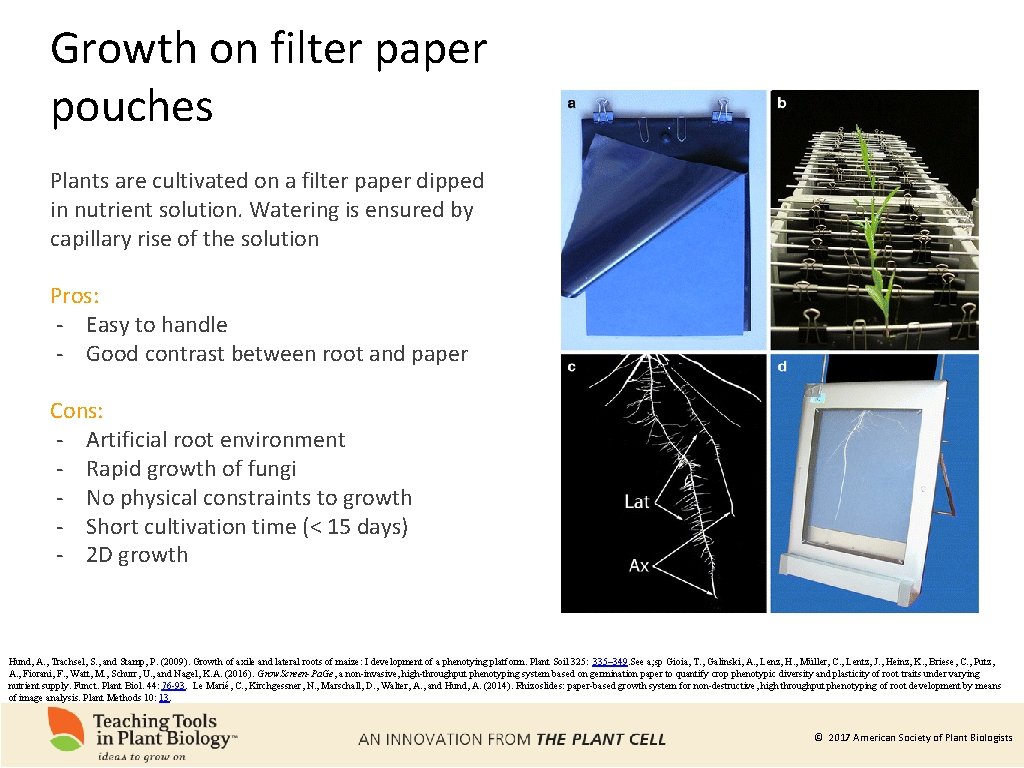
Growth on filter paper pouches Plants are cultivated on a filter paper dipped in nutrient solution. Watering is ensured by capillary rise of the solution Pros: - Easy to handle - Good contrast between root and paper Cons: - Artificial root environment - Rapid growth of fungi - No physical constraints to growth - Short cultivation time (< 15 days) - 2 D growth Hund, A. , Trachsel, S. , and Stamp, P. (2009). Growth of axile and lateral roots of maize: I development of a phenotying platform. Plant Soil 325: 335– 349. See a; sp Gioia, T. , Galinski, A. , Lenz, H. , Müller, C. , Lentz, J. , Heinz, K. , Briese, C. , Putz, A. , Fiorani, F. , Watt, M. , Schurr, U. , and Nagel, K. A. (2016). Grow. Screen-Pa. Ge, a non-invasive, high-throughput phenotyping system based on germination paper to quantify crop phenotypic diversity and plasticity of root traits under varying nutrient supply. Funct. Plant Biol. 44: 76 -93. Le Marié, C. , Kirchgessner, N. , Marschall, D. , Walter, A. , and Hund, A. (2014). Rhizoslides: paper-based growth system for non-destructive, high throughput phenotyping of root development by means of image analysis. Plant Methods 10: 13. © 2017 American Society of Plant Biologists

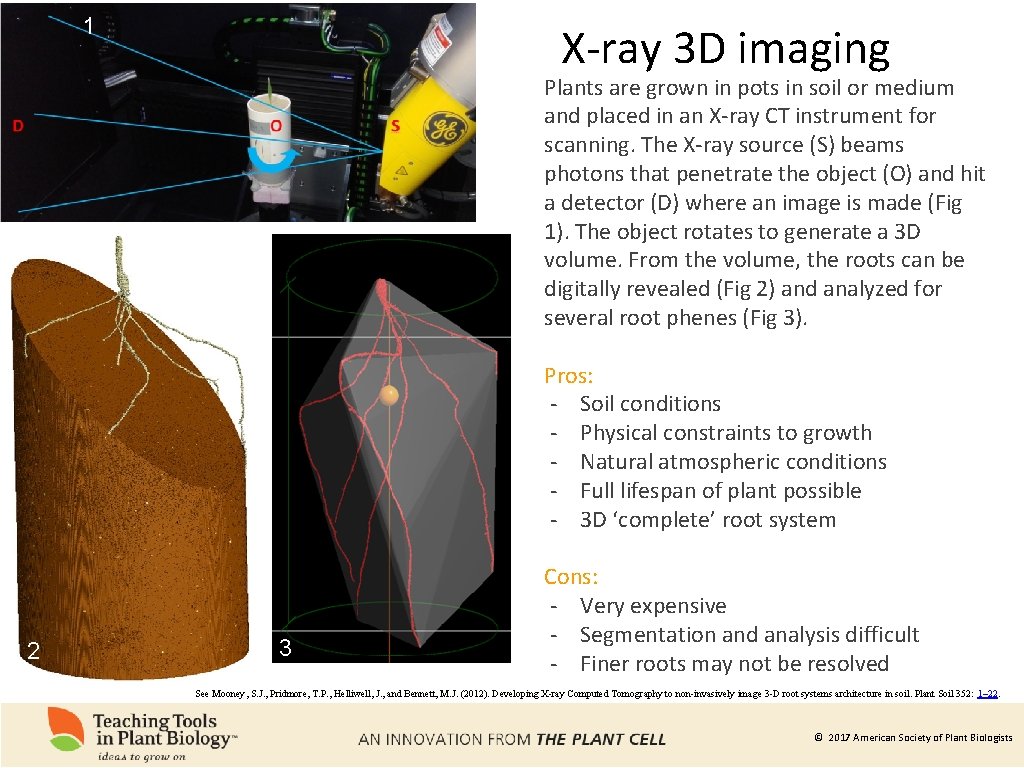
1 X-ray 3 D imaging Plants are grown in pots in soil or medium and placed in an X-ray CT instrument for scanning. The X-ray source (S) beams photons that penetrate the object (O) and hit a detector (D) where an image is made (Fig 1). The object rotates to generate a 3 D volume. From the volume, the roots can be digitally revealed (Fig 2) and analyzed for several root phenes (Fig 3). Pros: - Soil conditions - Physical constraints to growth - Natural atmospheric conditions - Full lifespan of plant possible - 3 D ‘complete’ root system 2 3 Cons: - Very expensive - Segmentation and analysis difficult - Finer roots may not be resolved See Mooney, S. J. , Pridmore, T. P. , Helliwell, J. , and Bennett, M. J. (2012). Developing X-ray Computed Tomography to non-invasively image 3 -D root systems architecture in soil. Plant Soil 352: 1– 22. © 2017 American Society of Plant Biologists

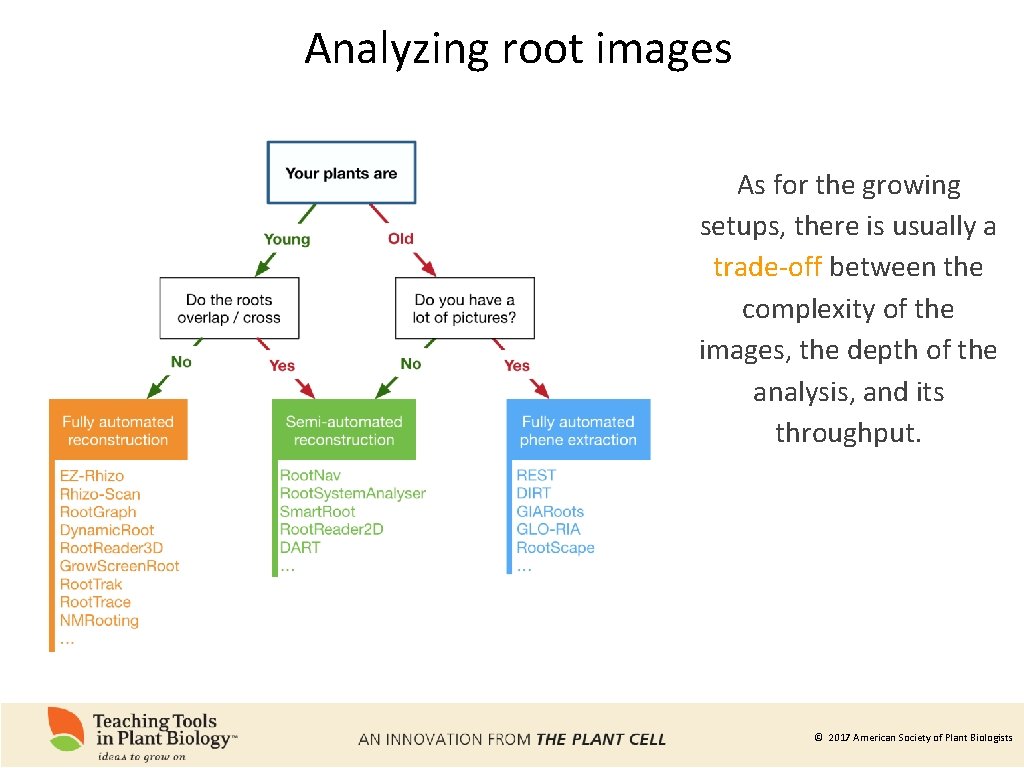
Analyzing root images As for the growing setups, there is usually a trade-off between the complexity of the images, the depth of the analysis, and its throughput. © 2017 American Society of Plant Biologists

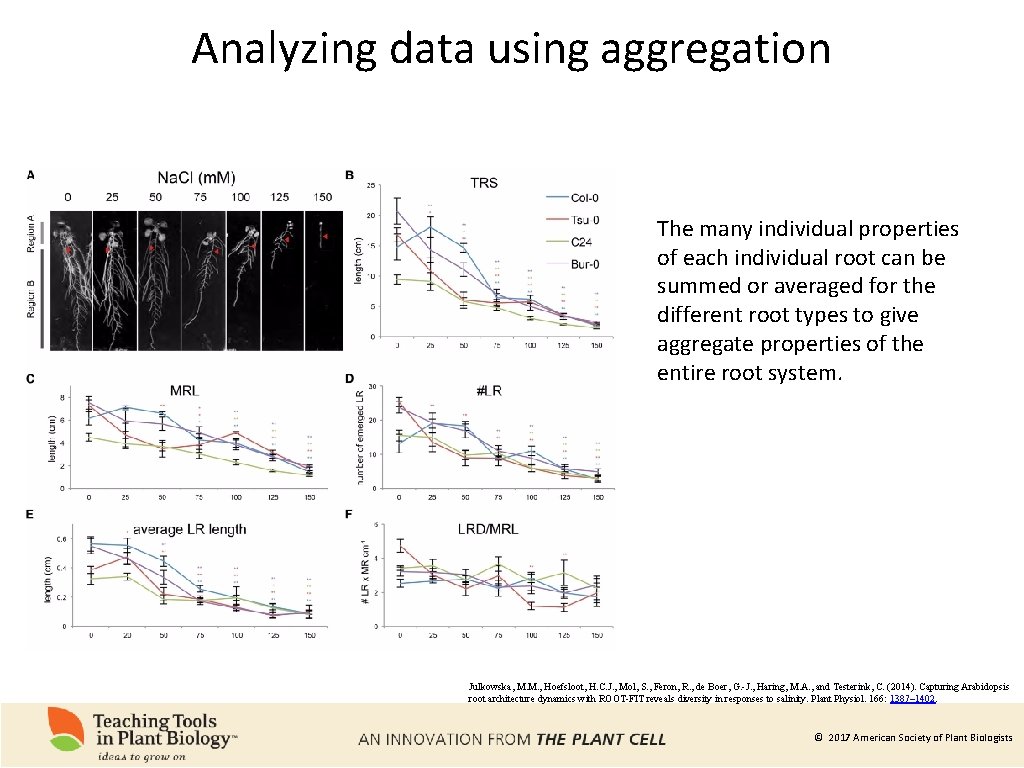
Analyzing data using aggregation The many individual properties of each individual root can be summed or averaged for the different root types to give aggregate properties of the entire root system. Julkowska, M. M. , Hoefsloot, H. C. J. , Mol, S. , Feron, R. , de Boer, G. -J. , Haring, M. A. , and Testerink, C. (2014). Capturing Arabidopsis root architecture dynamics with ROOT-FIT reveals diversity in responses to salinity. Plant Physiol. 166: 1387– 1402. © 2017 American Society of Plant Biologists

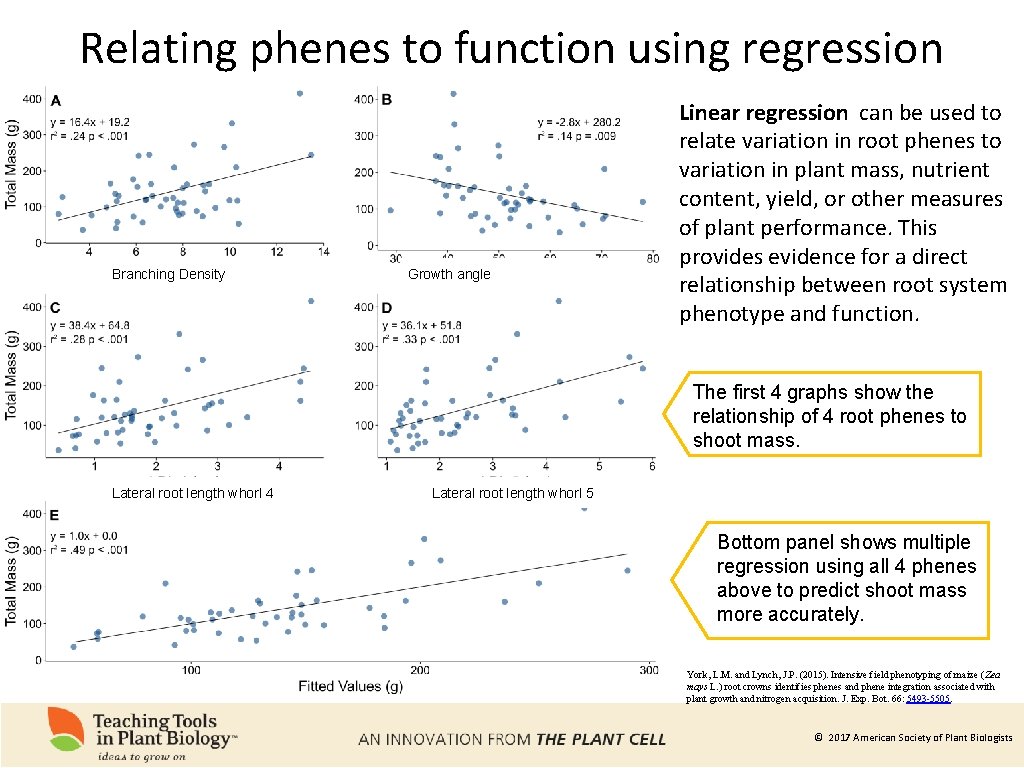
Relating phenes to function using regression Branching Density Growth angle Linear regression can be used to relate variation in root phenes to variation in plant mass, nutrient content, yield, or other measures of plant performance. This provides evidence for a direct relationship between root system phenotype and function. The first 4 graphs show the relationship of 4 root phenes to shoot mass. Lateral root length whorl 4 Lateral root length whorl 5 Bottom panel shows multiple regression using all 4 phenes above to predict shoot mass more accurately. York, L. M. and Lynch, J. P. (2015). Intensive field phenotyping of maize (Zea mays L. ) root crowns identifies phenes and phene integration associated with plant growth and nitrogen acquisition. J. Exp. Bot. 66: 5493 -5505. © 2017 American Society of Plant Biologists

Understanding how roots forage for soil resources is imperative for closing the yield gap (the gap between theoretical and actual yields) while the global population grows and climate changes. Therefore, acquiring root data quickly and accurately is an important challenge. This challenge is being met using various imaging approaches coupled to image analysis, borrowing from the fields of computer vision and machine learning. The phenomics of root system architecture will allow the integration of beneficial phene states in elite crop varieties to feed the world. © 2017 American Society of Plant Biologists