Phenomenological Model for Hydrocarbon HighTemperature Autoignition M Petrova

Phenomenological Model for Hydrocarbon High-Temperature Autoignition M. Petrova & F. Williams Domenic Tassoni
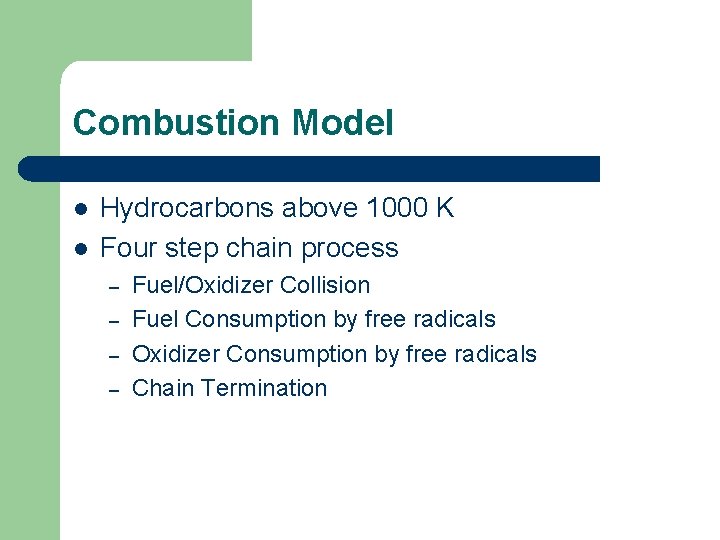
Combustion Model l l Hydrocarbons above 1000 K Four step chain process – – Fuel/Oxidizer Collision Fuel Consumption by free radicals Oxidizer Consumption by free radicals Chain Termination
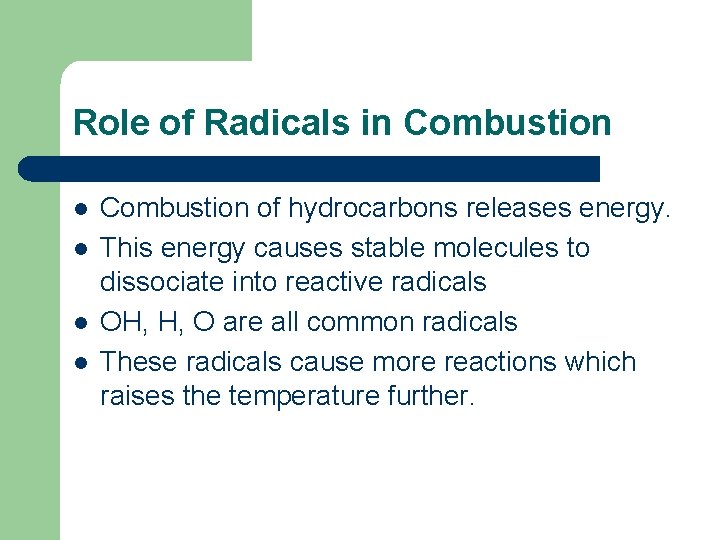
Role of Radicals in Combustion l l Combustion of hydrocarbons releases energy. This energy causes stable molecules to dissociate into reactive radicals OH, H, O are all common radicals These radicals cause more reactions which raises the temperature further.
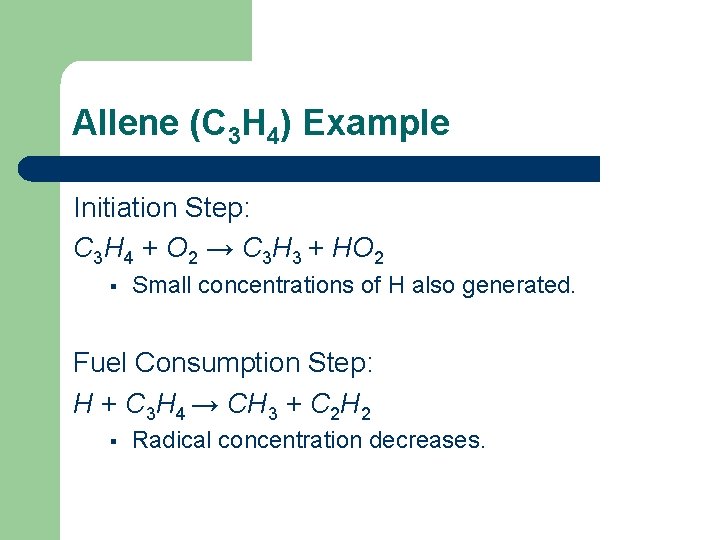
Allene (C 3 H 4) Example Initiation Step: C 3 H 4 + O 2 → C 3 H 3 + HO 2 § Small concentrations of H also generated. Fuel Consumption Step: H + C 3 H 4 → CH 3 + C 2 H 2 § Radical concentration decreases.
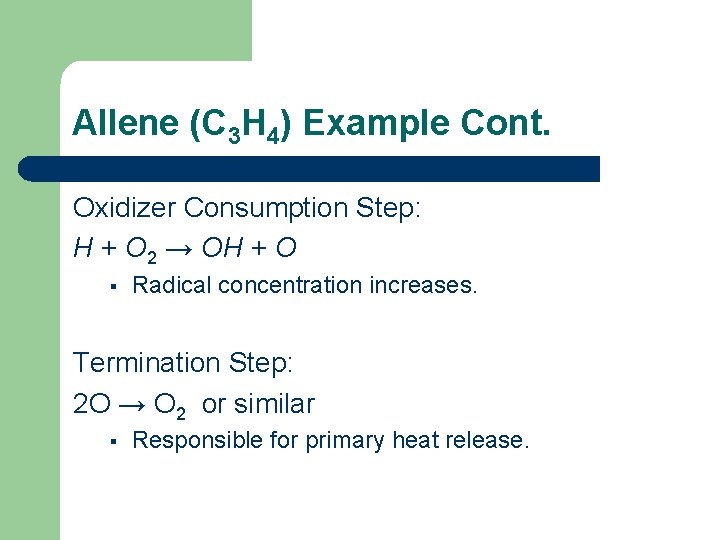
Allene (C 3 H 4) Example Cont. Oxidizer Consumption Step: H + O 2 → OH + O § Radical concentration increases. Termination Step: 2 O → O 2 or similar § Responsible for primary heat release.
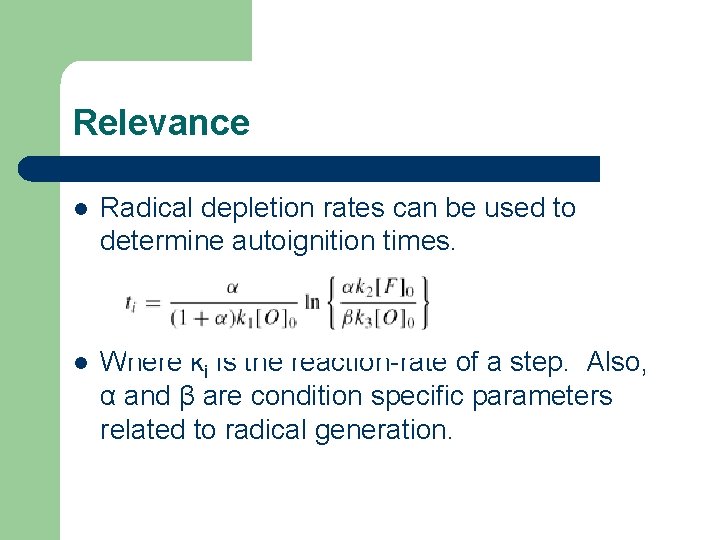
Relevance l Radical depletion rates can be used to determine autoignition times. l Where ki is the reaction-rate of a step. Also, α and β are condition specific parameters related to radical generation.
Other Possible Uses for the Model l Burning velocities Diffusion-flame extinction conditions Detonation cell sizes

Thank You l Questions?
- Slides: 8