Phases of matter Solids liquids and gases Matter



















- Slides: 25

Phases of matter Solids, liquids, and gases

Matter • Matter exists in 4 different forms, 3 of which we see and know about in every day life. • Solids, liquids, and gases are the primary phases of mater that we encounter daily • Plasma is the 4 th, but we only see it as lightning and stars. • The different phases of matter are the result of varying intermolecular forces and bonds.

Kinetic theory of gases • Kinetic theory states that atoms are in constant random motion, having a certain amount of energy that is dependent on factors such as molecular weight, temperature, and pressure. • Intermolecular forces overcome the random chaotic motion of atoms to create the various phases of matter. Without intermolecular forces all matter would exist in the gas phase

• The stronger the intermolecular forces of a molecule, the less freedom the molecules will have • Temperature of the molecules also plays a large role in the movement of molecules. As temperature drops atoms and molecules lose energy, and intermolecular forces start to take hold. • The energy a molecule has is directly dependent on the molecule in question. • https: //phet. colorado. edu/en/simulation/stat es-of-matter-basics

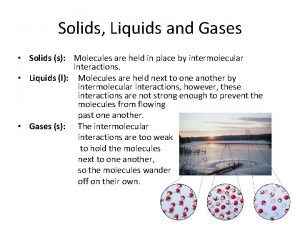
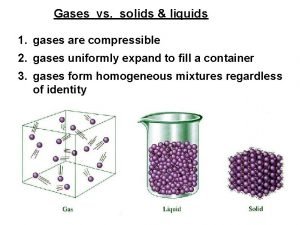
Solids • Solids are the most dense form of matter. Solids are rigid and cannot be compressed easily • Atoms are packed closely together and are unable to move past one another. • Solids hold their shape and have a fixed volume • Solids have the strongest effect of intermolecular forces.

Liquids • Liquids are relatively dense matter that cannot be easily compressed. • Liquids do not have a definite shape but do have a definite volume. Liquids will take the shape of their container. • Liquid molecules are in constant random motion moving around each other unlike solids, but cannot completely leave the group of molecules.

Gases • Gases are the least dense form of matter • Gases take the shape and volume of the container that they are in. • 1 gas molecule will do its very best to take the shape of its container no matter how large or what the shape is. • Gases are highly compressible fluids, where individual atoms or molecules are able to move freely around one another. Gases have a lot of empty space in them.

Phase changes • When matter changes from one phase to another it is predictable given certain parameters and certain mathematical values can be determined. • 6 phase changes are involved in the transformation of matter. • Condensing, freezing, melting, vaporization, sublimation, deposition.

vaporizing and condensing • • The transformation between liquid and gas. Vaporizing = liquid to gas Condensing = gas to liquid The vaporization point and condensation point are the same temperature for a substance.

Evaporation & boiling • Evaporation and boiling are types of vaporization. • Evaporation occurs at any temperature but only at the surface. Some particles in a liquid are moving faster than the others and move to the surface. At the surface, there are half as many intermolecular force interactions, leading to the molecule or atoms easily breaking off. • Boiling occurs throughout the liquid. The point at which the vapor pressure of a liquid equals or exceeds the pressure exerted on the liquid (usually atmospheric pressure).

Melting and freezing • Melting and freezing is the change between a liquid and a solid. • Melting = Solid to liquid • Freezing = Liquid to solid • The melting and freezing point of a substance is the same.

Sublimation and deposition Changing between a gas and a solid. Deposition = Gas to a solid Sublimation = Solid to gas. Sublimation and deposition have the same temperature value for a given substance. • Sublimation and deposition are not common in nature. Examples: sublimation – Dry ice, deposition – hoar frost • •


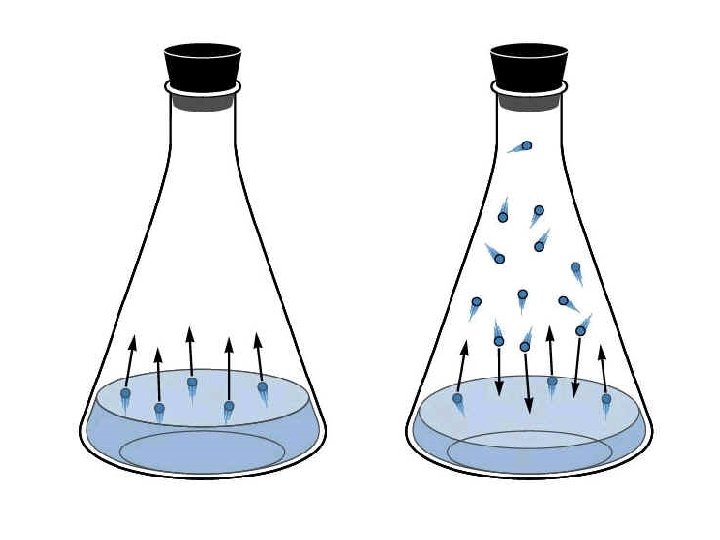
Vapor pressure • Liquids and some solids are continuously vaporizing. • Molecules moving in a liquid have a certain amount of energy. As a molecule moves to the surface of a liquid, it will only escape into the gas phase if they have enough energy to overcome the attractions between the molecules that hold the liquid together.

• Vapor pressure is the partial pressure of the vapor of a substance above the liquid, measured at equilibrium at a certain temperature, usually measured in mm of mercury. • Think of vapor pressure as the strength that a gas pushes with above its liquid. • Once a certain amount of liquid has reached the gas phase in a container no more will vaporize, and equilibrium is established.


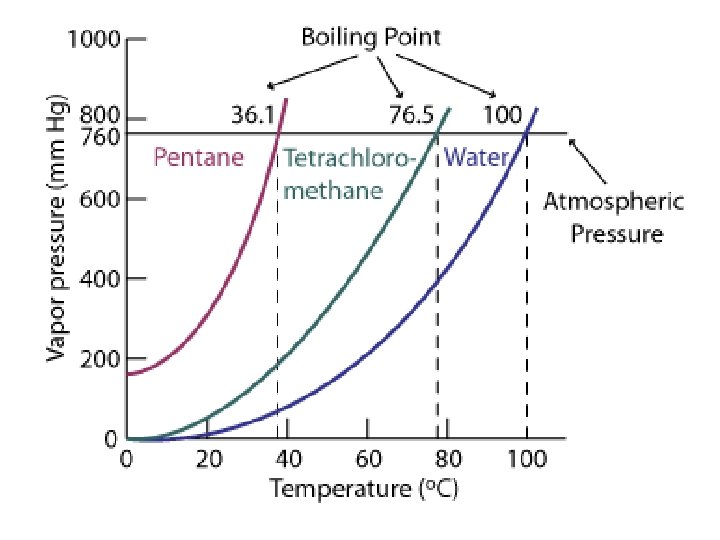
Vapor pressure - temperature • Vapor pressure is dependent on the temperature of the system. • As temperature increases, the vapor pressure of the liquid increases. • A volatile liquid is something that has a high vapor pressure at a low temperature. • https: //www. youtube. com/watch? v=Jbqtq. Cu n. Yz. A


Boiling • When heat is added to a liquid, the energy of the molecules increases. When the energy of the molecules increases the vapor pressure increases • When the vapor pressure of a liquid exeeds the external pressure of the liquid bubbles will begin the form and the liquid begins to boil. • The temperature at which this happens is the boiling point of the liquid.

Boiling - pressure • Boiling point is dependent on the external pressure exerted on the liquid. At sea level, the atmospheric pressure is 1 atm, and the boiling point of water is 100*C. • But at a higher elevation, for example denver colorado, the atmospheric pressure is 0. 83 atm, and water boils at 95*C.

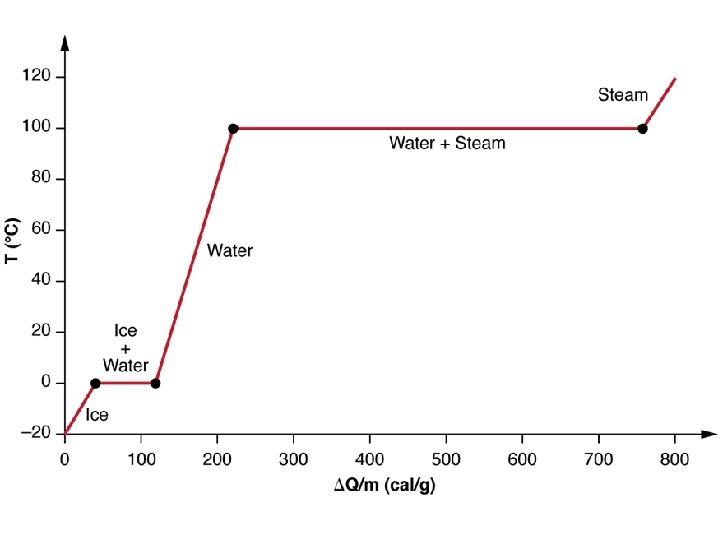
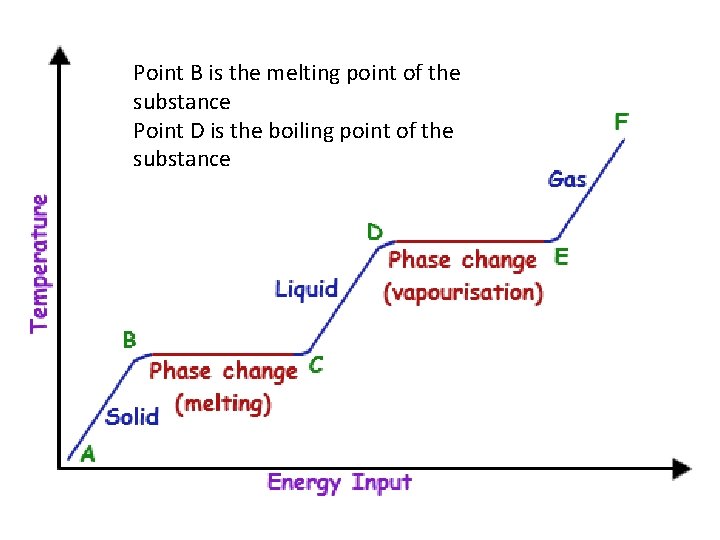
Phase change diagrams • Diagrams can be created for any substance that plot the temperature change of a substance. • Certain characteristics of a phase change diagram let you determine the temperatures at which the substance will boil and melt.


• During a phase change, the temperature of a substance will increase until it reaches a melting or boiling point. • When a substance begins to melt, the temperature will not continue to rise until all of the substance has changed to the new phase.

Point B is the melting point of the substance Point D is the boiling point of the substance

Energy • The amount of energy needed to melt a solid is called the heat of fusion • The amount of energy needed to vaporize a liquid is called the heat of vaporization.
 Thermal expansion and contraction examples
Thermal expansion and contraction examples Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids States of matter diagram
States of matter diagram Mass of solid liquid and gas
Mass of solid liquid and gas Examples of solids liquids and gases pictures
Examples of solids liquids and gases pictures Lesson 1 thermal energy and the behavior of matter
Lesson 1 thermal energy and the behavior of matter Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kinetic molecular theory of solid
Kinetic molecular theory of solid Red liquid element
Red liquid element How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Matter and its composition
Matter and its composition Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Why is gas easier to compress than a liquid or a solid
Why is gas easier to compress than a liquid or a solid Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Gas to solid
Gas to solid Adhesive force
Adhesive force Liquids and solids menu
Liquids and solids menu Kesler science.com
Kesler science.com Molecular theory of gases and liquids
Molecular theory of gases and liquids Filtering solids from liquids
Filtering solids from liquids Four phases of matter
Four phases of matter 4 phases of matter
4 phases of matter 5 phases of matter
5 phases of matter 3 phases of matter
3 phases of matter Why isn't it a good idea to classify matter by its phases
Why isn't it a good idea to classify matter by its phases States of matter foldable
States of matter foldable