Phase Transformations Growth Kinetics ByeongJoo Lee POSTECH MSE

Phase Transformations Growth Kinetics Byeong-Joo Lee POSTECH - MSE calphad@postech. ac. kr Byeong-Joo Lee cmse. postech. ac. kr
General Background ※ References: 1. W. D. Kingery, H. K. Bowen and D. R. Uhlmann, "Introduction to Ceramics", John Wiley & Sons. Chap. 8. 2. Christian, section 56 & 54. 3. J. Burke, "The Kinetics of Phase Transformations in Metals, " Pergamon Press. Chap. 6. Byeong-Joo Lee cmse. postech. ac. kr
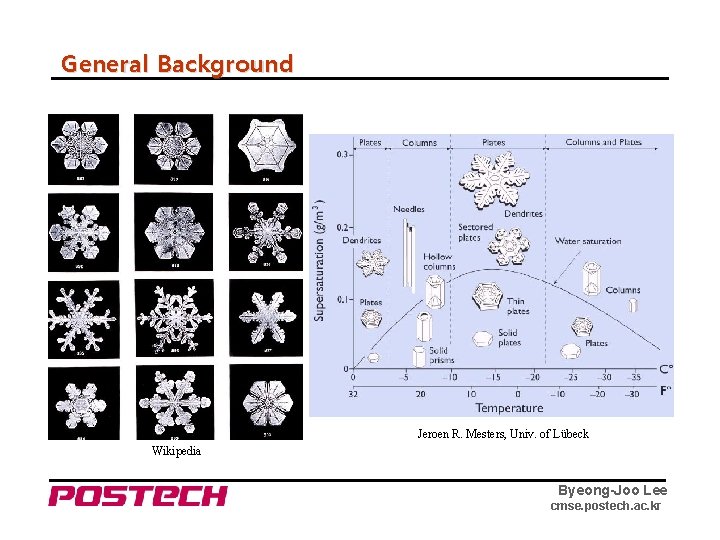
General Background Jeroen R. Mesters, Univ. of Lübeck Wikipedia Byeong-Joo Lee cmse. postech. ac. kr
General Background Byeong-Joo Lee cmse. postech. ac. kr
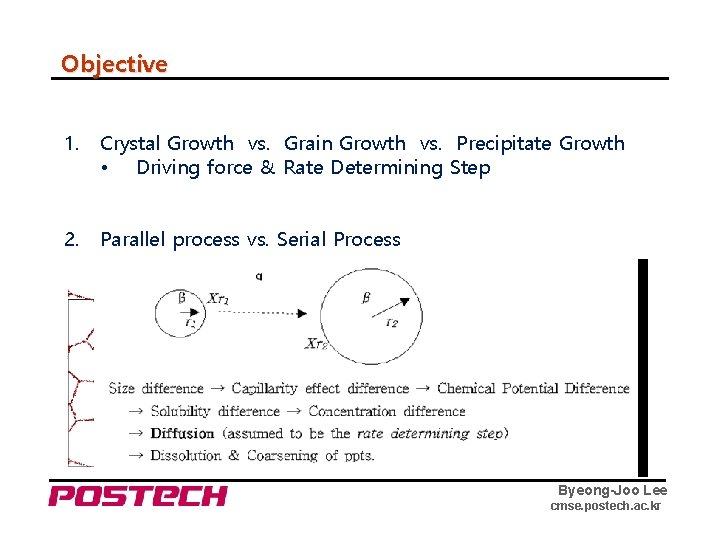
Objective 1. Crystal Growth vs. Grain Growth vs. Precipitate Growth • Driving force & Rate Determining Step 2. Parallel process vs. Serial Process 3. Interface Reaction vs. Diffusion Controlled Process 4. Interface: Continuous Growth vs. Lateral Growth Byeong-Joo Lee cmse. postech. ac. kr
Classification of Growth Process - Interface-Reaction Controlled Growth □ Interface-Reaction Controlled Growth ▷ Changes which do not involve long-range diffusional transport ex) growth of a pure solid grain growth - curvature driven kinetics recrystallization massive transformation martensitic transformation antiphase domain coarsening order-disorder transformation ※ Even phase transformations that involve composition changes may be interface-reaction limited. - local equilibrium is not applied at the interface. Byeong-Joo Lee cmse. postech. ac. kr

Classification of Growth Process - Diffusion Controlled Growth □ Diffusion Controlled Growth ▷ Changes which involve long-range diffusional transport ▷ Assumptions ․ local equilibrium at the interface : the concentration on either side of the interface is given by the phase diagram ※ for conditions under which this assumption might break down, see: Langer & Sekerka, Acta Metall. 23, 1225 (1975). ․ capillarity effects are ignored. ․ the diffusion coefficient is frequently assumed to be independent from concentration. Byeong-Joo Lee cmse. postech. ac. kr

Interface-Reaction Controlled Growth - Mechanism □ Two types of IRC growth mechanism - Continuous growth and growth by a lateral migration of steps Continuous growth can only occur when the boundary is unstable with respect to motion normal to itself. - It can add material across the interface at all points with equal ease. - Comparison of the two mechanisms Continuous Growth disordered interface diffuse interface high driving force Lateral motion of steps ordered/singular interface sharp interface low driving force Byeong-Joo Lee cmse. postech. ac. kr
Interface-Reaction Controlled Growth - Growth of a pure Solid ex) single crystal growth during solidification or deposition ▷ Continuous growth reaction rate in a thermally activated process (in Chemical Reaction Kinetics) ⇒ (ν/RT)·exp (-ΔG*/RT)·ΔGdf a thermally activated migration of grain boundaries ⇒ v = M·ΔGdf for example, for solidification ⇒ v = k 1․ΔTi Byeong-Joo Lee cmse. postech. ac. kr
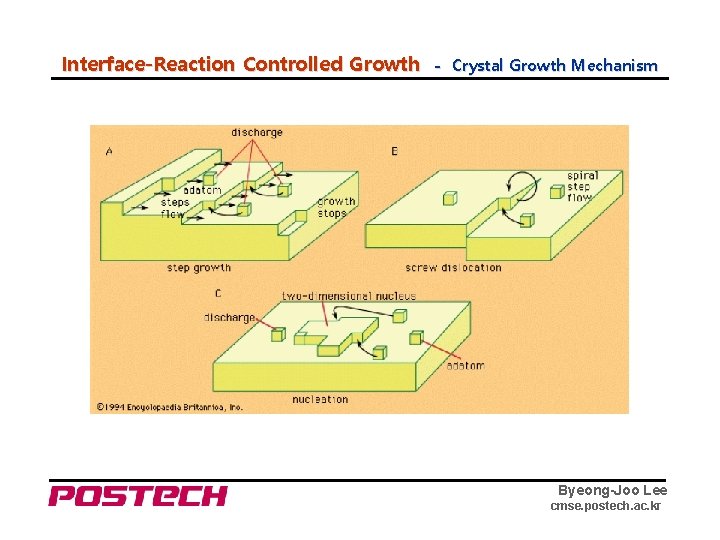
Interface-Reaction Controlled Growth - Crystal Growth Mechanism Byeong-Joo Lee cmse. postech. ac. kr

Interface-Reaction Controlled Growth - Growth of a pure Solid ▷ Lateral growth ex) solidification of materials with a high entropy of melting minimum free energy ⇔ minimum number of broken bond source of ledge of jog : (i) surface nucleation (ii) spiral growth (iii) twin boundary (i) surface nucleation : two-dimensional homogeneous nucleation problem existence of critical nucleus size, r* the growth rate normal to the interface ∝ nucleation rate ⇒ v ∝ exp ( - k 2 /ΔTi ) (ii) spiral growth : ⇒ v = k 3·(ΔTi)2 (iii) twin boundary : similar to the spiral growth mechanism Byeong-Joo Lee cmse. postech. ac. kr

Byeong-Joo Lee cmse. postech. ac. kr
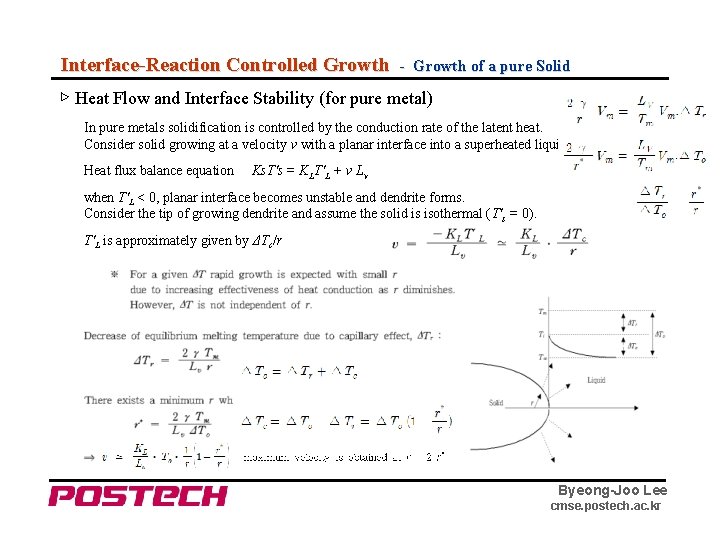
Interface-Reaction Controlled Growth - Growth of a pure Solid ▷ Heat Flow and Interface Stability (for pure metal) In pure metals solidification is controlled by the conduction rate of the latent heat. Consider solid growing at a velocity v with a planar interface into a superheated liquid. Heat flux balance equation Ks. T's = KLT'L + v Lv when T'L < 0, planar interface becomes unstable and dendrite forms. Consider the tip of growing dendrite and assume the solid is isothermal (T's = 0). T'L is approximately given by ΔTc/r Byeong-Joo Lee cmse. postech. ac. kr
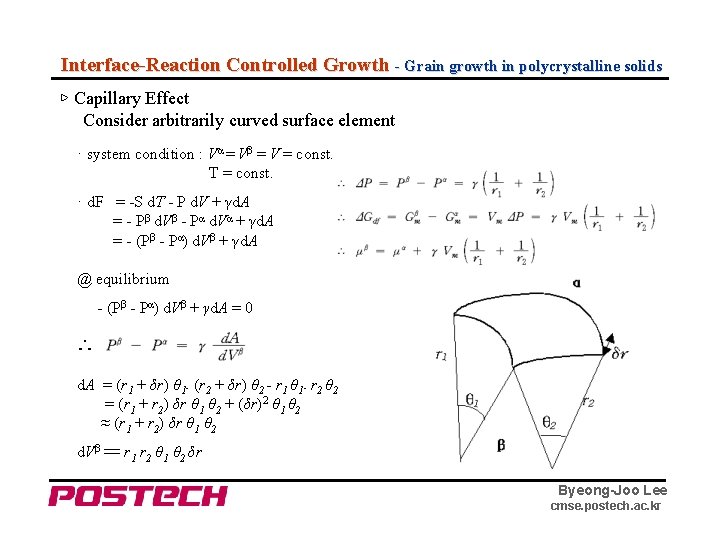
Interface-Reaction Controlled Growth - Grain growth in polycrystalline solids ▷ Capillary Effect Consider arbitrarily curved surface element · system condition : Vα = Vβ = V = const. T = const. · d. F = -S d. T - P d. V + γd. A = - Pβ d. Vβ - Pα d. Vα + γd. A = - (Pβ - Pα) d. Vβ + γd. A @ equilibrium - (Pβ - Pα) d. Vβ + γd. A = 0 ∴ d. A = (r 1 + δr) θ 1․(r 2 + δr) θ 2 - r 1 θ 1․r 2 θ 2 = (r 1 + r 2) δr θ 1 θ 2 + (δr)2 θ 1 θ 2 ≈ (r 1 + r 2) δr θ 1 θ 2 d. Vβ 〓 r 1 r 2 θ 1 θ 2 δr Byeong-Joo Lee cmse. postech. ac. kr
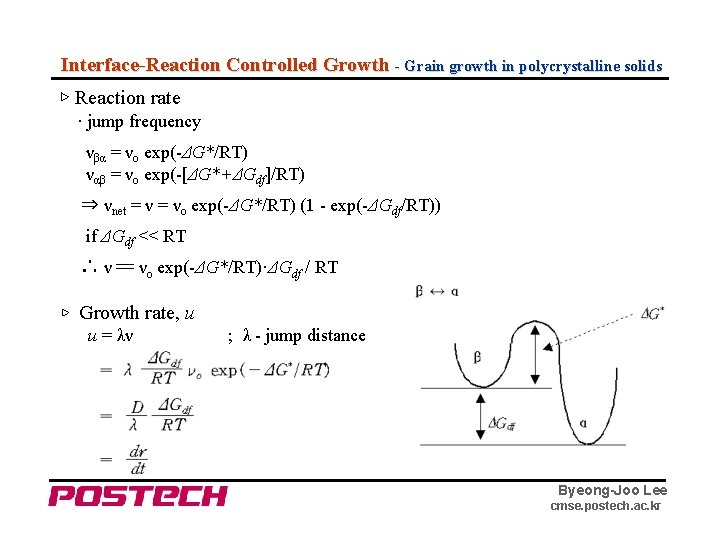
Interface-Reaction Controlled Growth - Grain growth in polycrystalline solids ▷ Reaction rate · jump frequency νβα = νo exp(-ΔG*/RT) ναβ = νo exp(-[ΔG*+ΔGdf]/RT) ⇒ νnet = νo exp(-ΔG*/RT) (1 - exp(-ΔGdf/RT)) if ΔGdf << RT ∴ ν 〓 νo exp(-ΔG*/RT)·ΔGdf / RT ▷ Growth rate, u u = λν ; λ - jump distance Byeong-Joo Lee cmse. postech. ac. kr
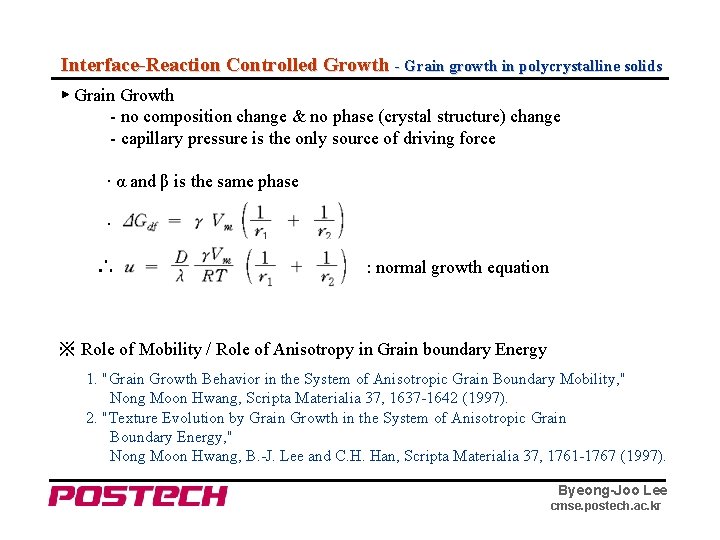
Interface-Reaction Controlled Growth - Grain growth in polycrystalline solids ▶ Grain Growth - no composition change & no phase (crystal structure) change - capillary pressure is the only source of driving force · α and β is the same phase · ∴ : normal growth equation ※ Role of Mobility / Role of Anisotropy in Grain boundary Energy 1. "Grain Growth Behavior in the System of Anisotropic Grain Boundary Mobility, " Nong Moon Hwang, Scripta Materialia 37, 1637 -1642 (1997). 2. "Texture Evolution by Grain Growth in the System of Anisotropic Grain Boundary Energy, " Nong Moon Hwang, B. -J. Lee and C. H. Han, Scripta Materialia 37, 1761 -1767 (1997). Byeong-Joo Lee cmse. postech. ac. kr
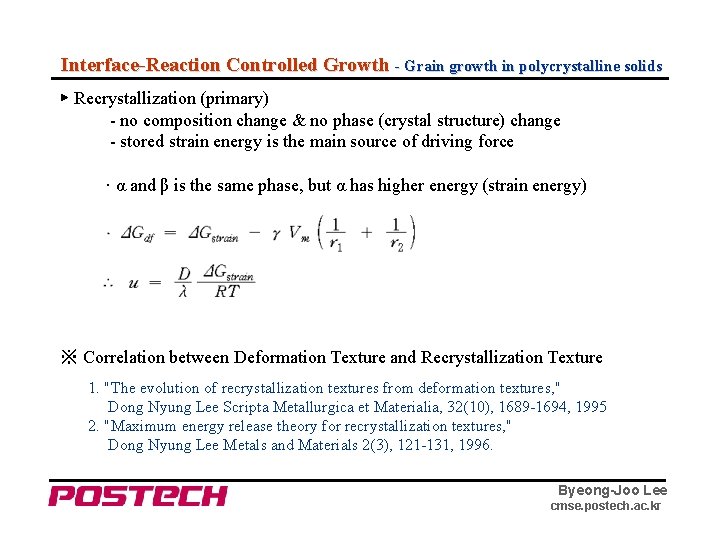
Interface-Reaction Controlled Growth - Grain growth in polycrystalline solids ▶ Recrystallization (primary) - no composition change & no phase (crystal structure) change - stored strain energy is the main source of driving force · α and β is the same phase, but α has higher energy (strain energy) ※ Correlation between Deformation Texture and Recrystallization Texture 1. "The evolution of recrystallization textures from deformation textures, " Dong Nyung Lee Scripta Metallurgica et Materialia, 32(10), 1689 -1694, 1995 2. "Maximum energy release theory for recrystallization textures, " Dong Nyung Lee Metals and Materials 2(3), 121 -131, 1996. Byeong-Joo Lee cmse. postech. ac. kr
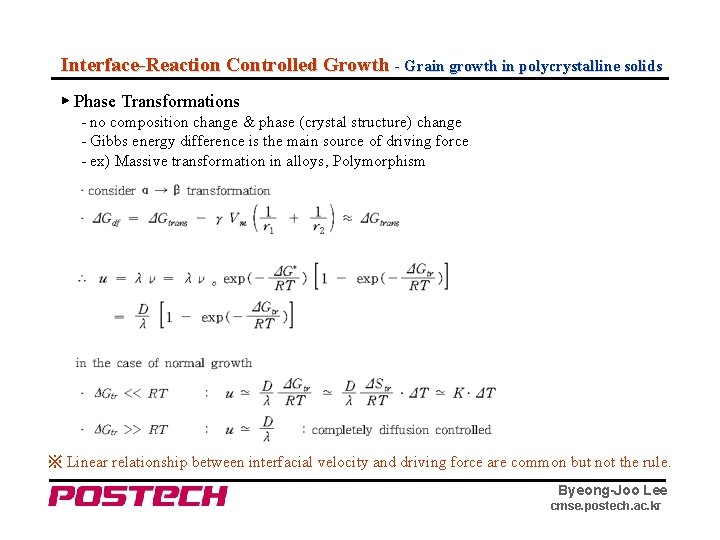
Interface-Reaction Controlled Growth - Grain growth in polycrystalline solids ▶ Phase Transformations - no composition change & phase (crystal structure) change - Gibbs energy difference is the main source of driving force - ex) Massive transformation in alloys, Polymorphism ※ Linear relationship between interfacial velocity and driving force are common but not the rule. Byeong-Joo Lee cmse. postech. ac. kr
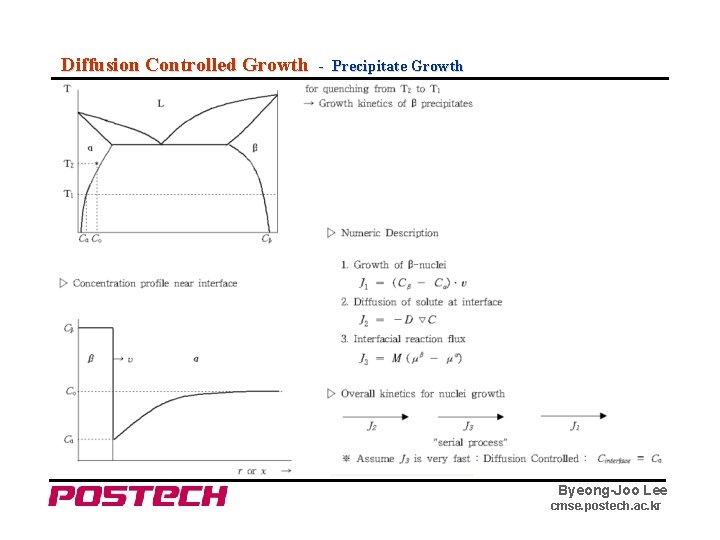
Diffusion Controlled Growth - Precipitate Growth Byeong-Joo Lee cmse. postech. ac. kr
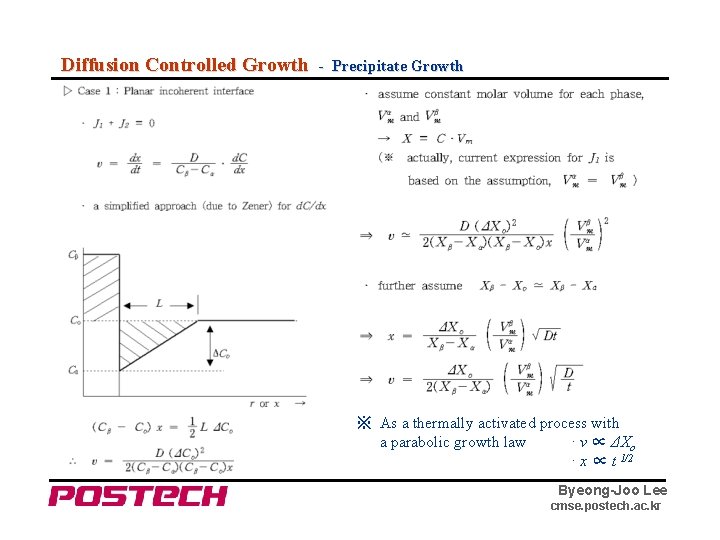
Diffusion Controlled Growth - Precipitate Growth ※ As a thermally activated process with a parabolic growth law · v ∝ ΔXo · x ∝ t 1/2 Byeong-Joo Lee cmse. postech. ac. kr
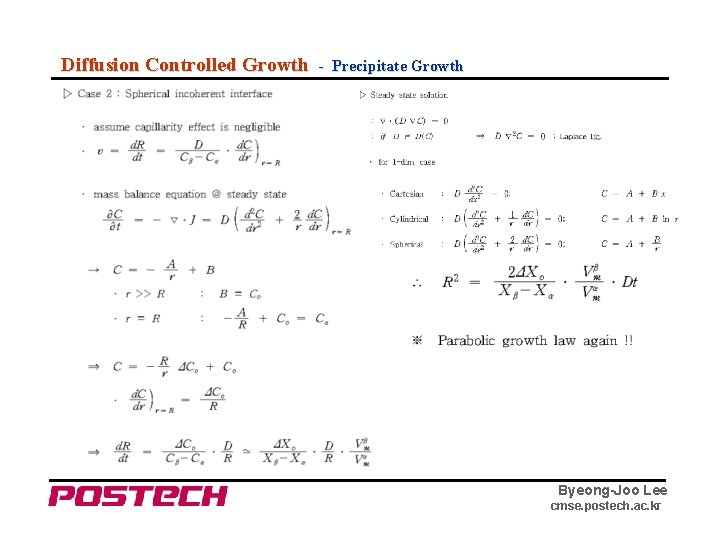
Diffusion Controlled Growth - Precipitate Growth Byeong-Joo Lee cmse. postech. ac. kr
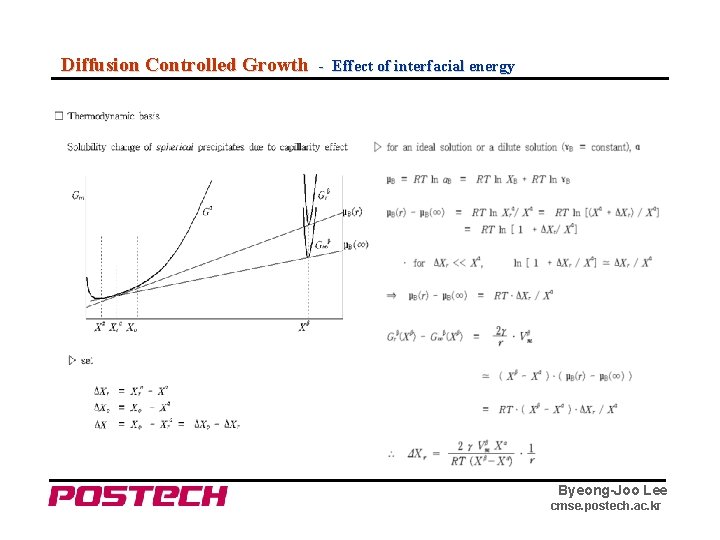
Diffusion Controlled Growth - Effect of interfacial energy Byeong-Joo Lee cmse. postech. ac. kr
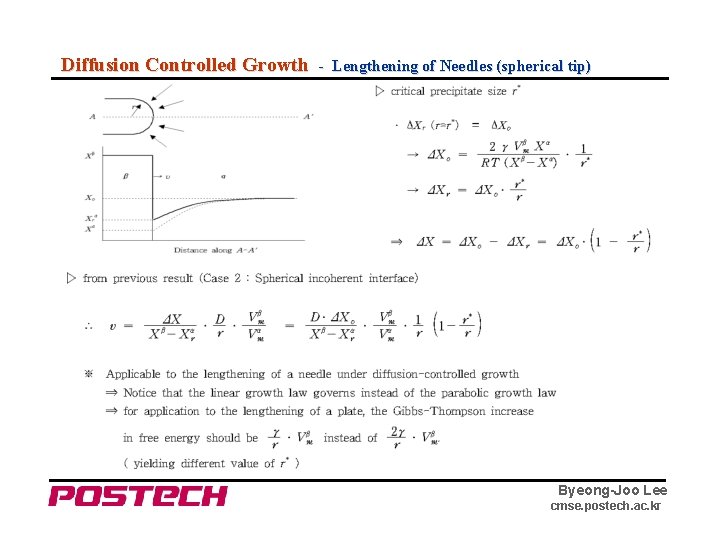
Diffusion Controlled Growth - Lengthening of Needles (spherical tip) Byeong-Joo Lee cmse. postech. ac. kr
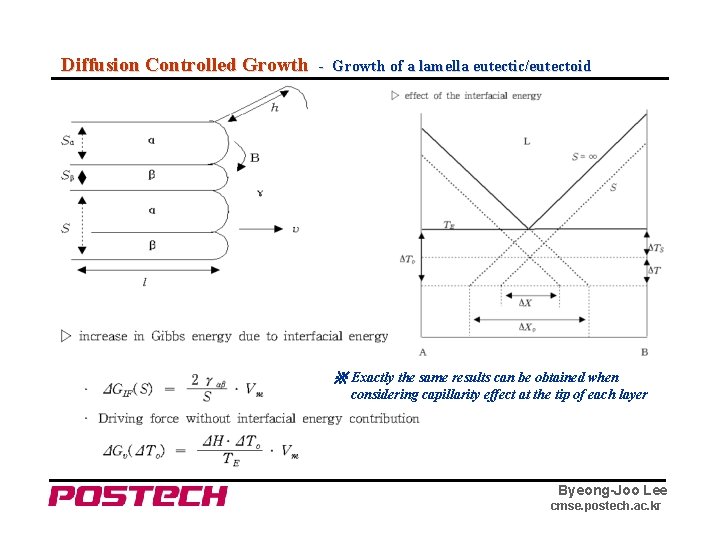
Diffusion Controlled Growth - Growth of a lamella eutectic/eutectoid ※ Exactly the same results can be obtained when considering capillarity effect at the tip of each layer Byeong-Joo Lee cmse. postech. ac. kr
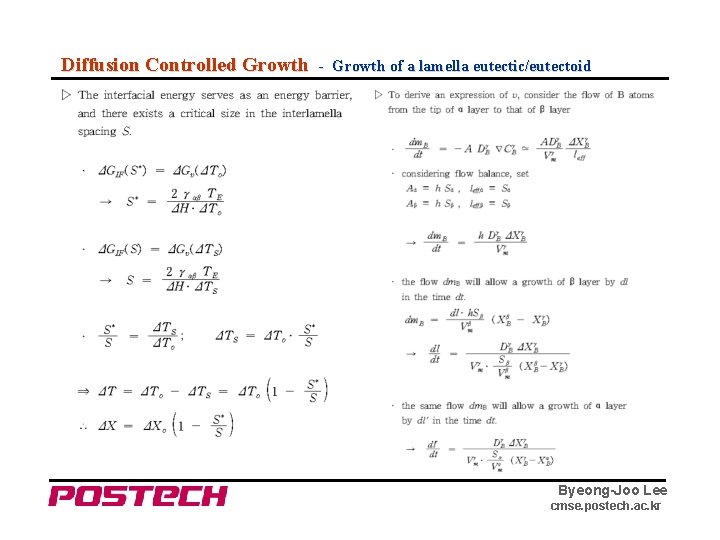
Diffusion Controlled Growth - Growth of a lamella eutectic/eutectoid Byeong-Joo Lee cmse. postech. ac. kr
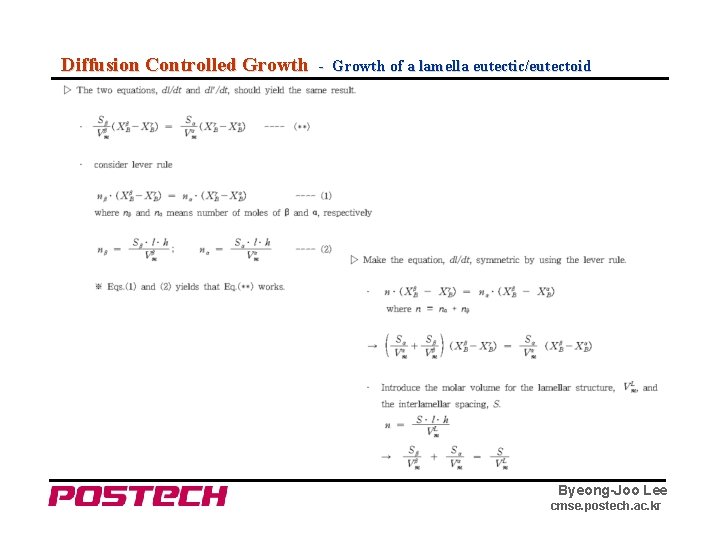
Diffusion Controlled Growth - Growth of a lamella eutectic/eutectoid Byeong-Joo Lee cmse. postech. ac. kr

Diffusion Controlled Growth - Growth of a lamella eutectic/eutectoid Byeong-Joo Lee cmse. postech. ac. kr
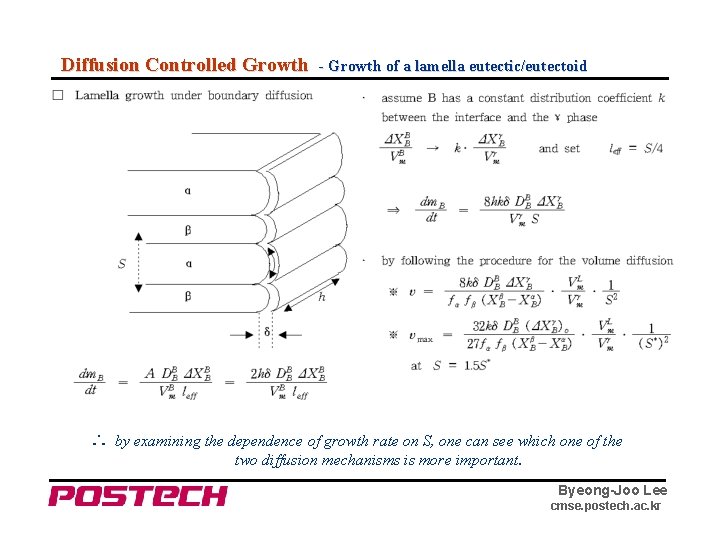
Diffusion Controlled Growth - Growth of a lamella eutectic/eutectoid ∴ by examining the dependence of growth rate on S, one can see which one of the two diffusion mechanisms is more important. Byeong-Joo Lee cmse. postech. ac. kr
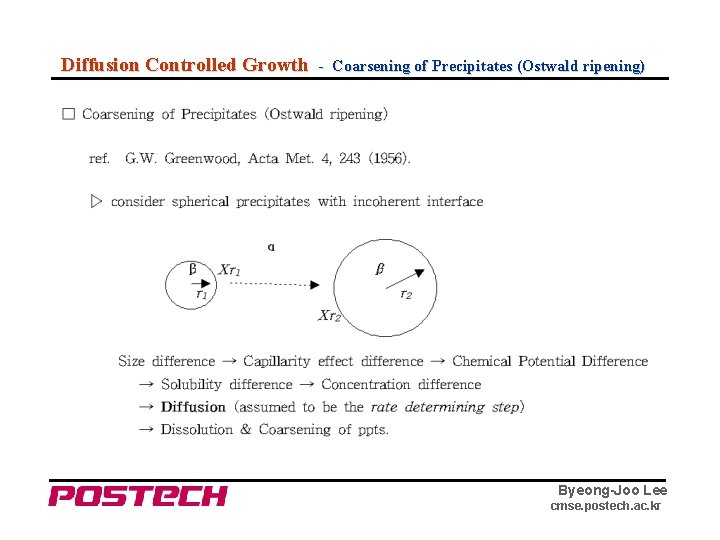
Diffusion Controlled Growth - Coarsening of Precipitates (Ostwald ripening) Byeong-Joo Lee cmse. postech. ac. kr
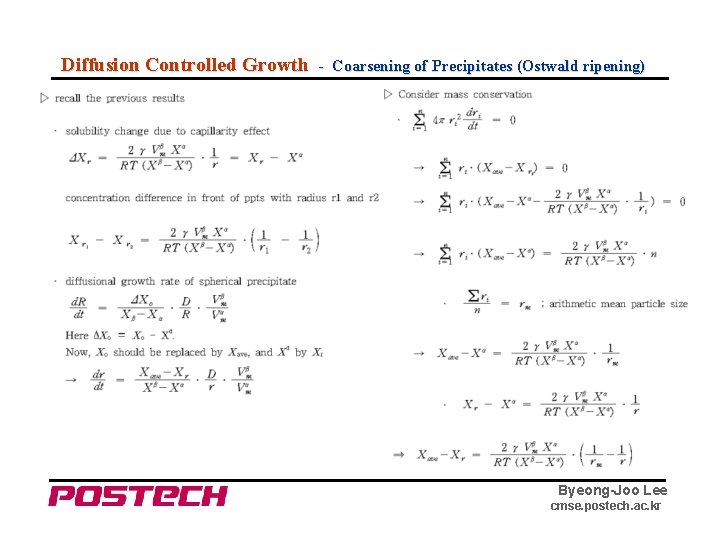
Diffusion Controlled Growth - Coarsening of Precipitates (Ostwald ripening) Byeong-Joo Lee cmse. postech. ac. kr
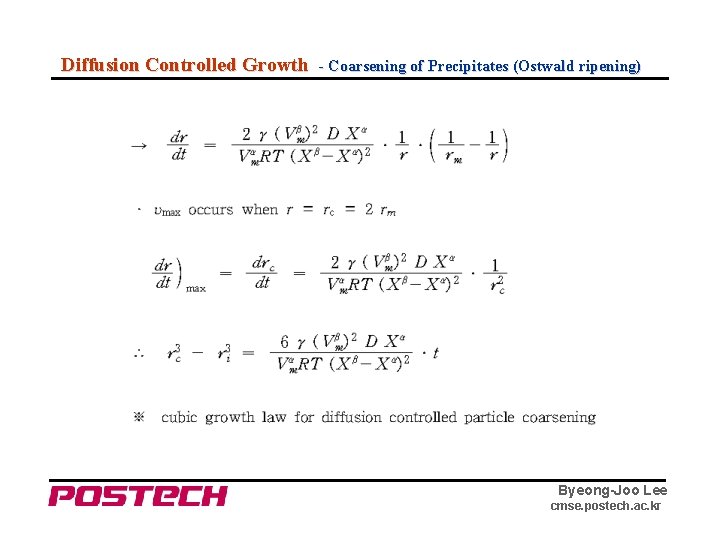
Diffusion Controlled Growth - Coarsening of Precipitates (Ostwald ripening) Byeong-Joo Lee cmse. postech. ac. kr
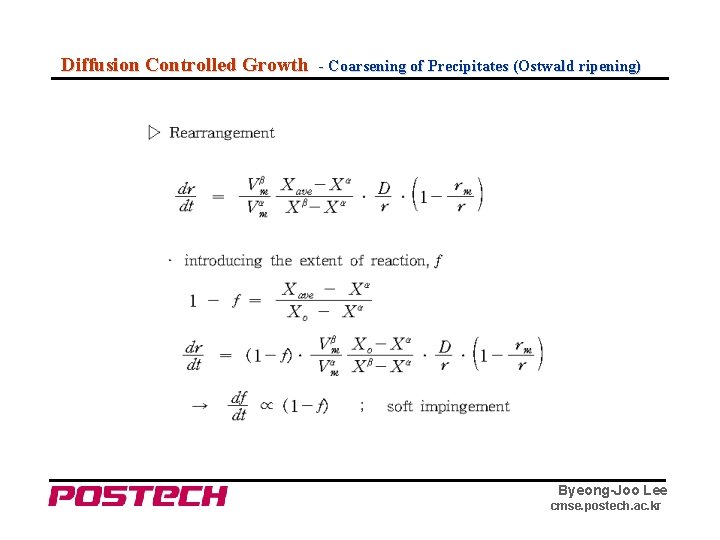
Diffusion Controlled Growth - Coarsening of Precipitates (Ostwald ripening) Byeong-Joo Lee cmse. postech. ac. kr
- Slides: 32