Phase Separation of WaterGlycerol Binary Mixtures Next to

Phase Separation of Water/Glycerol Binary Mixtures Next to Lipid Monolayers: An X-ray and Neutron Reflectivity Study Luka Pocivavsek James Franck Institute and Department of Chemistry, The University of Chicago, Chicago IL, 60637 USA
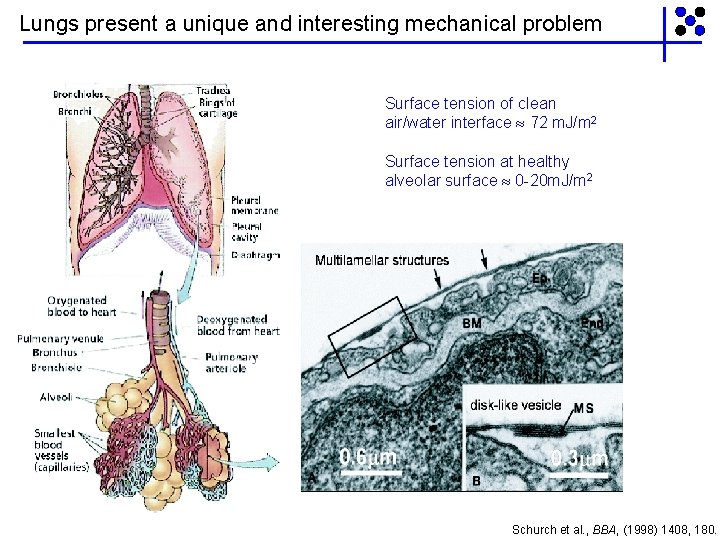
Lungs present a unique and interesting mechanical problem Surface tension of clean air/water interface 72 m. J/m 2 Surface tension at healthy alveolar surface 0 -20 m. J/m 2 Schurch et al. , BBA, (1998) 1408, 180.
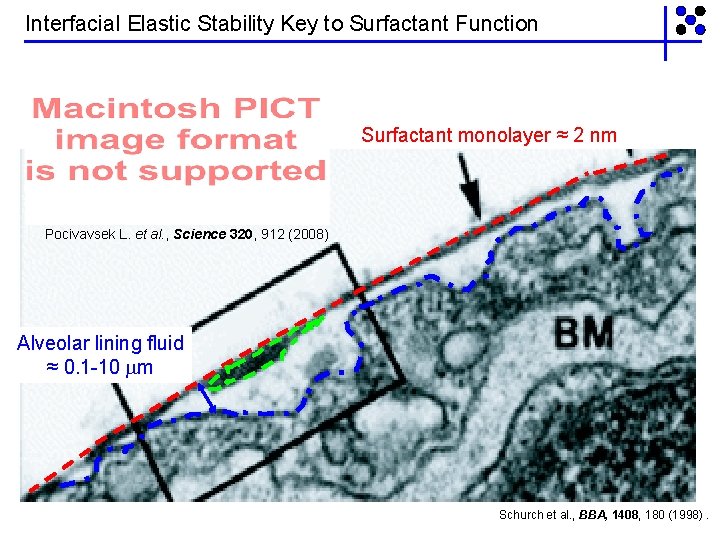
Interfacial Elastic Stability Key to Surfactant Function Surfactant monolayer ≈ 2 nm Pocivavsek L. et al. , Science 320, 912 (2008) Alveolar lining fluid ≈ 0. 1 -10 m Schurch et al. , BBA, 1408, 180 (1998).
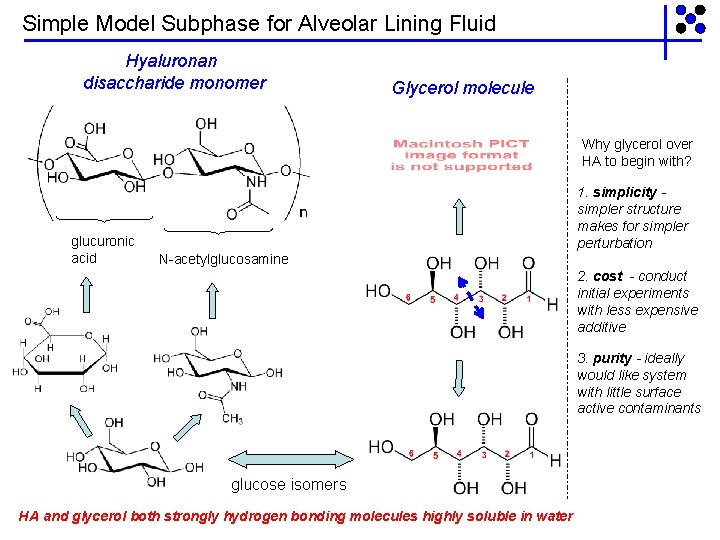
Simple Model Subphase for Alveolar Lining Fluid Hyaluronan disaccharide monomer Glycerol molecule Why glycerol over HA to begin with? glucuronic acid 1. simplicity simpler structure makes for simpler perturbation N-acetylglucosamine 2. cost - conduct initial experiments with less expensive additive 3. purity - ideally would like system with little surface active contaminants glucose isomers HA and glycerol both strongly hydrogen bonding molecules highly soluble in water
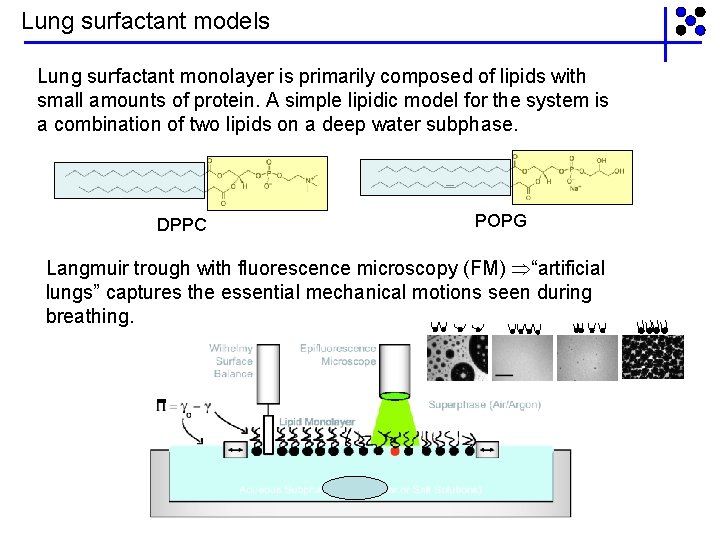
Lung surfactant models Lung surfactant monolayer is primarily composed of lipids with small amounts of protein. A simple lipidic model for the system is a combination of two lipids on a deep water subphase. DPPC POPG Langmuir trough with fluorescence microscopy (FM) “artificial lungs” captures the essential mechanical motions seen during breathing.
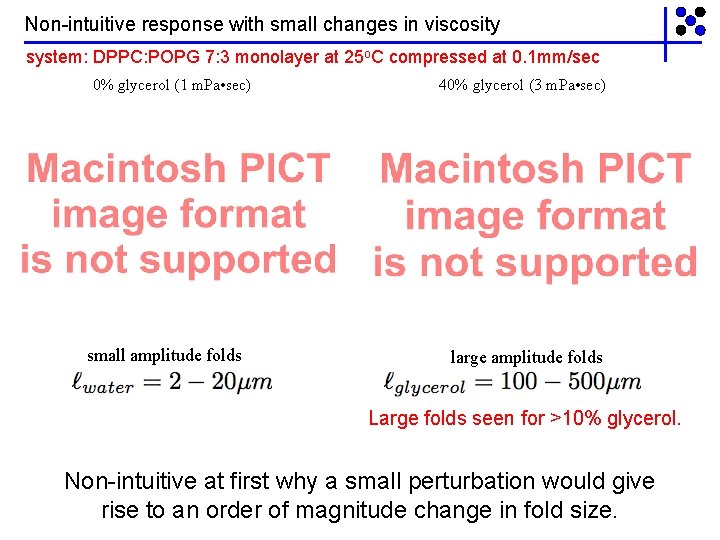
Non-intuitive response with small changes in viscosity system: DPPC: POPG 7: 3 monolayer at 25 o. C compressed at 0. 1 mm/sec 0% glycerol (1 m. Pa • sec) small amplitude folds 40% glycerol (3 m. Pa • sec) large amplitude folds Large folds seen for >10% glycerol. Non-intuitive at first why a small perturbation would give rise to an order of magnitude change in fold size.
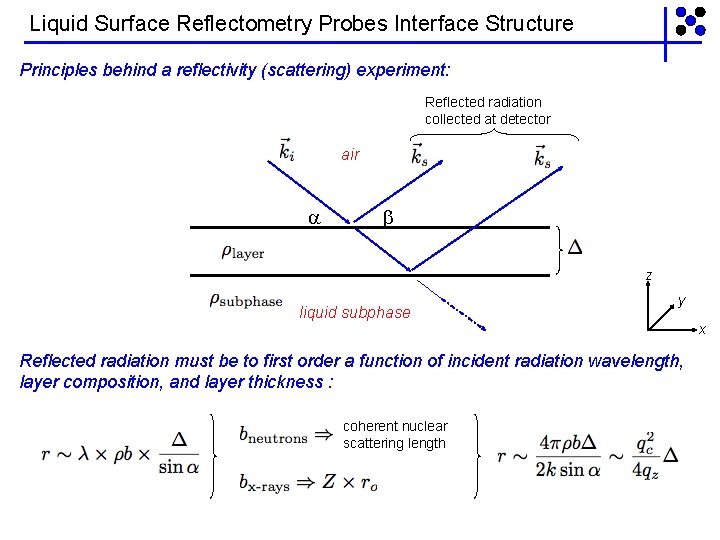
Liquid Surface Reflectometry Probes Interface Structure Principles behind a reflectivity (scattering) experiment: Reflected radiation collected at detector air z liquid subphase y x Reflected radiation must be to first order a function of incident radiation wavelength, layer composition, and layer thickness : coherent nuclear scattering length
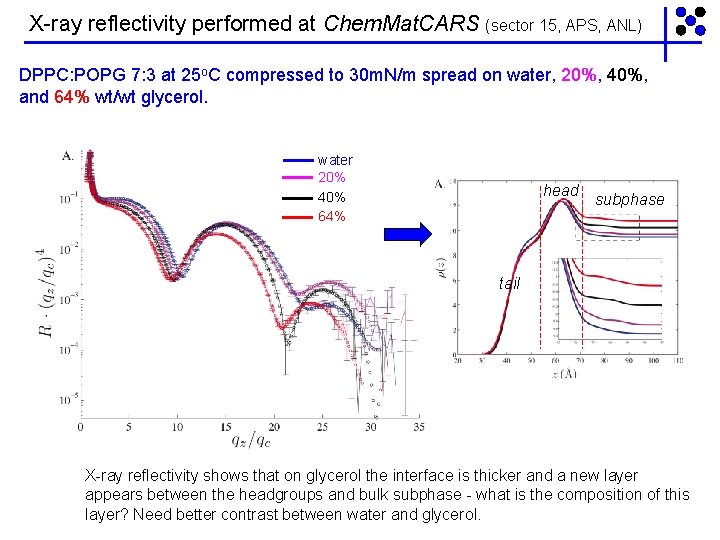
X-ray reflectivity performed at Chem. Mat. CARS (sector 15, APS, ANL) DPPC: POPG 7: 3 at 25 o. C compressed to 30 m. N/m spread on water, 20%, 40%, and 64% wt/wt glycerol. water 20% 40% 64% head subphase tail X-ray reflectivity shows that on glycerol the interface is thicker and a new layer appears between the headgroups and bulk subphase - what is the composition of this layer? Need better contrast between water and glycerol.
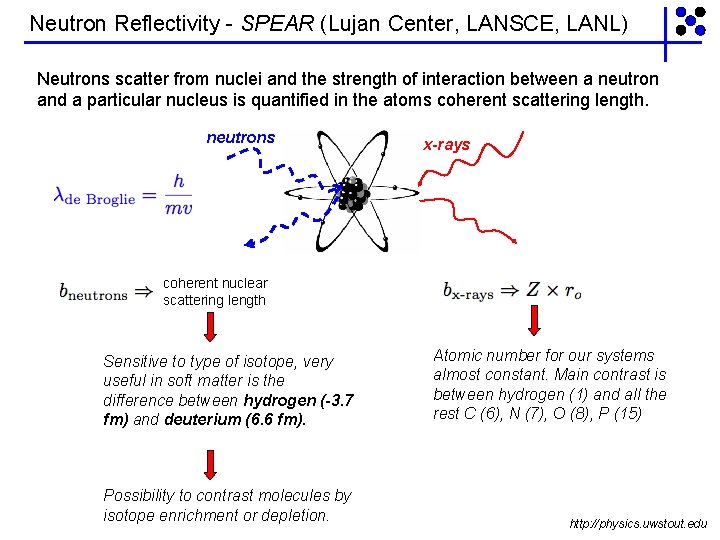
Neutron Reflectivity - SPEAR (Lujan Center, LANSCE, LANL) Neutrons scatter from nuclei and the strength of interaction between a neutron and a particular nucleus is quantified in the atoms coherent scattering length. neutrons x-rays coherent nuclear scattering length Sensitive to type of isotope, very useful in soft matter is the difference between hydrogen (-3. 7 fm) and deuterium (6. 6 fm). Possibility to contrast molecules by isotope enrichment or depletion. Atomic number for our systems almost constant. Main contrast is between hydrogen (1) and all the rest C (6), N (7), O (8), P (15) http: //physics. uwstout. edu
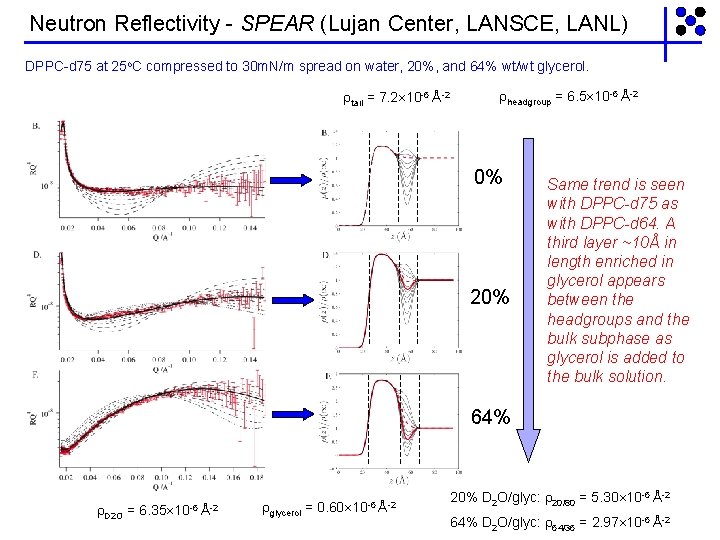
Neutron Reflectivity - SPEAR (Lujan Center, LANSCE, LANL) DPPC-d 75 at 25 o. C compressed to 30 m. N/m spread on water, 20%, and 64% wt/wt glycerol. tail = 7. 2 10 -6 Å-2 headgroup = 6. 5 10 -6 Å-2 0% 20% Same trend is seen with DPPC-d 75 as with DPPC-d 64. A third layer ~10Å in length enriched in glycerol appears between the headgroups and the bulk subphase as glycerol is added to the bulk solution. 64% D 2 O = 6. 35 10 -6 Å-2 glycerol = 0. 60 10 -6 Å-2 20% D 2 O/glyc: 20/80 = 5. 30 10 -6 Å-2 64% D 2 O/glyc: 64/36 = 2. 97 10 -6 Å-2
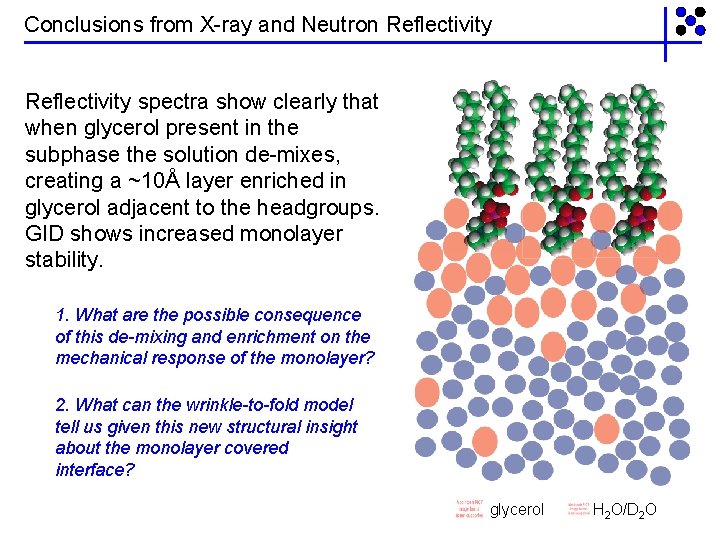
Conclusions from X-ray and Neutron Reflectivity spectra show clearly that when glycerol present in the subphase the solution de-mixes, creating a ~10Å layer enriched in glycerol adjacent to the headgroups. GID shows increased monolayer stability. 1. What are the possible consequence of this de-mixing and enrichment on the mechanical response of the monolayer? 2. What can the wrinkle-to-fold model tell us given this new structural insight about the monolayer covered interface? glycerol H 2 O/D 2 O
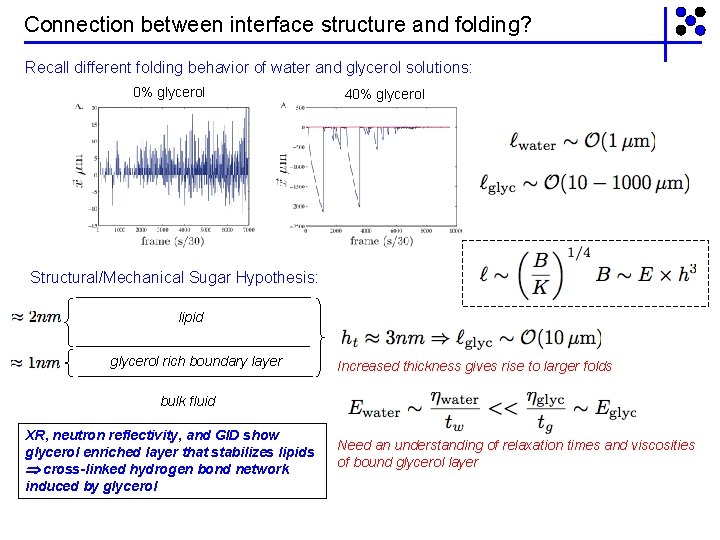
Connection between interface structure and folding? Recall different folding behavior of water and glycerol solutions: 0% glycerol 40% glycerol Structural/Mechanical Sugar Hypothesis: lipid glycerol rich boundary layer Increased thickness gives rise to larger folds bulk fluid XR, neutron reflectivity, and GID show glycerol enriched layer that stabilizes lipids cross-linked hydrogen bond network induced by glycerol Need an understanding of relaxation times and viscosities of bound glycerol layer
Acknowledgments Ka Yee Lee, Enrique Cerda, Binhua Lin, Jarek Majewski University of Chicago Tom Witten Kseniya Gavrilov Eva Chi Kathleen Cao Shelli Frey Dongxu Li Brian Leahy USACH/Ude. C Sebastian Johnson Nico Rivas SPEAR, Lujan, LANL Jarek Majewski Eric Watkins Chem. Mat. CARS, ANL/UChicago Binhua Lin Mati Meron Tim Graber Jim Viccaro
- Slides: 13