Phase I Trial of Mosunetuzumab Bispecific Antibody in

Phase I Trial of Mosunetuzumab Bispecific Antibody in Multiply Relapsed Follicular Lymphoma CCO Independent Conference Highlights of the 2020 Virtual ASH Annual Meeting, December 5 -8, 2020 *CCO is an independent medical education company that provides state-of-the-art medical information to healthcare professionals through conference coverage and other educational programs. Supported by educational grants from Amgen; Astra. Zeneca; Bristol-Myers Squibb; Epizyme, Inc. ; Glaxo. Smith. Kline; Incyte Corporation; Janssen Biotech; Karyopharm Therapeutics Inc. ; Novartis; Pharma. Essentia Corp. ; Seattle Genetics; and Takeda Oncology.
About These Slides § Please feel free to use, update, and share some or all of these slides in your noncommercial presentations to colleagues or patients § When using our slides, please retain the source attribution: Slide credit: clinicaloptions. com § These slides may not be published, posted online, or used in commercial presentations without permission. Please contact permissions@clinicaloptions. com for details
Mosunetuzumab in R/R FL: Background § Relapse is common in FL, despite multiple therapeutic advances; each relapse characterized by shorter duration of response, increasing refractoriness to therapy, and a poor prognosis[1] § Relapsed high-risk FL can be divided into subgroups based on time to relapse and refractoriness to previous therapy[2]: ‒ Progression of disease within 24 mos of frontline treatment ‒ Double refractoriness to an anti-CD 20 antibody and an alkylating agent § Mosunetuzumab is a humanized Ig. G 1 anti-CD 20 x CD 3 bispecific antibody, which engages and redirects T cells to eliminate malignant B cells[3] § This analysis of the phase I/Ib (GO 29781) dose-escalation trial of mosunetuzumab in R/R B-cell NHL reports updated clinical data for the FL cohort[3] 1. Batlevi. Blood Cancer J. 2020; 10: 74. 2. Casulo. Blood. 2017; 133: 1540. 3. Assouline. ASH 2020. Abstr 702. Slide credit: clinicaloptions. com
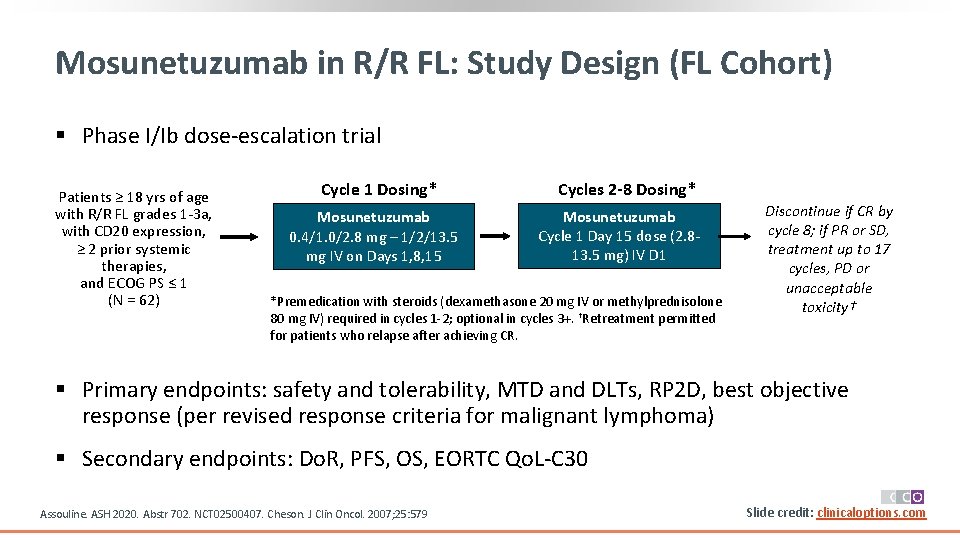
Mosunetuzumab in R/R FL: Study Design (FL Cohort) § Phase I/Ib dose-escalation trial Patients ≥ 18 yrs of age with R/R FL grades 1 -3 a, with CD 20 expression, ≥ 2 prior systemic therapies, and ECOG PS ≤ 1 (N = 62) Cycle 1 Dosing* Mosunetuzumab 0. 4/1. 0/2. 8 mg – 1/2/13. 5 mg IV on Days 1, 8, 15 Cycles 2 -8 Dosing* Mosunetuzumab Cycle 1 Day 15 dose (2. 813. 5 mg) IV D 1 *Premedication with steroids (dexamethasone 20 mg IV or methylprednisolone 80 mg IV) required in cycles 1 -2; optional in cycles 3+. †Retreatment permitted for patients who relapse after achieving CR. Discontinue if CR by cycle 8; if PR or SD, treatment up to 17 cycles, PD or unacceptable toxicity† § Primary endpoints: safety and tolerability, MTD and DLTs, RP 2 D, best objective response (per revised response criteria for malignant lymphoma) § Secondary endpoints: Do. R, PFS, OS, EORTC Qo. L-C 30 Assouline. ASH 2020. Abstr 702. NCT 02500407. Cheson. J Clin Oncol. 2007; 25: 579 Slide credit: clinicaloptions. com
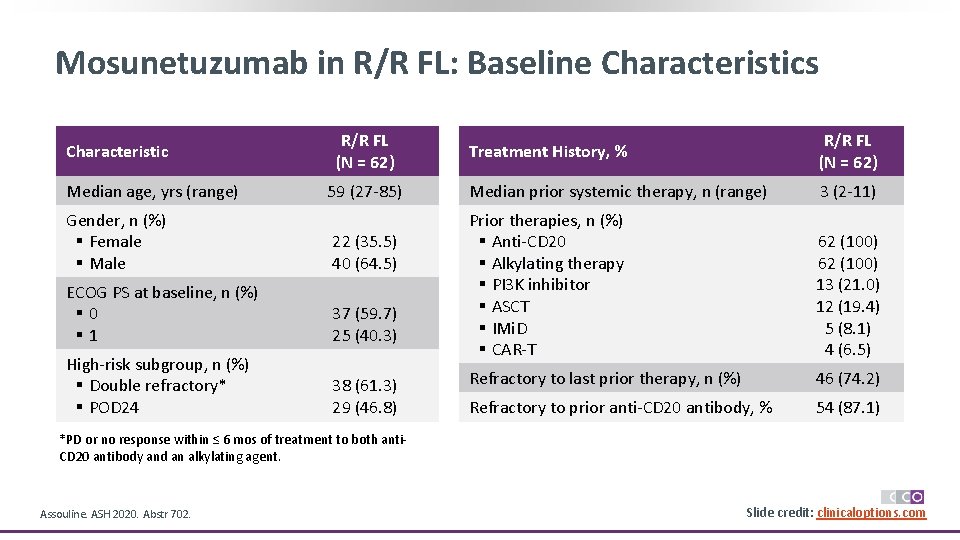
Mosunetuzumab in R/R FL: Baseline Characteristics Characteristic Median age, yrs (range) R/R FL (N = 62) 59 (27 -85) Gender, n (%) § Female § Male 22 (35. 5) 40 (64. 5) ECOG PS at baseline, n (%) § 0 § 1 37 (59. 7) 25 (40. 3) High-risk subgroup, n (%) § Double refractory* § POD 24 38 (61. 3) 29 (46. 8) Treatment History, % R/R FL (N = 62) Median prior systemic therapy, n (range) 3 (2 -11) Prior therapies, n (%) § Anti-CD 20 § Alkylating therapy § PI 3 K inhibitor § ASCT § IMi. D § CAR-T 62 (100) 13 (21. 0) 12 (19. 4) 5 (8. 1) 4 (6. 5) Refractory to last prior therapy, n (%) 46 (74. 2) Refractory to prior anti-CD 20 antibody, % 54 (87. 1) *PD or no response within ≤ 6 mos of treatment to both anti. CD 20 antibody and an alkylating agent. Assouline. ASH 2020. Abstr 702. Slide credit: clinicaloptions. com

Mosunetuzumab in R/R FL: Efficacy Response in Patient Subgroups n ORR, % CR, % PR, % All FL patients 62 67. 7 51. 6 16. 1 46 65. 2 47. 8 17. 4 38 29 71. 1 75. 9 50. 0 55. 2 21. 1 20. 7 PI 3 K inhibitor refractory 13 92. 3 84. 6 7. 7 Prior CAR T-cell therapy 4 100 50 50 High risk § Refractory to last prior therapy § Double refractory § POD 24 § Consistent responses across high-risk populations (double refractory; POD 24; PI 3 K refractory; prior CAR T-cell therapy) Assouline. ASH 2020. Abstr 702. § Antitumor activity (ORR: 67. 7%; CR: 51. 6%) observed across all dose levels § Median follow-up after first response: 18. 4 mos (range: 2 -34) § Median Do. R: 20. 4 mos (95% CI: 9. 4 -22. 7) § Do. R in patients with CR: 21. 0 mos (95% CI: 16. 0 -22. 7) § 4 patients received retreatment after relapse following CR ‒ n = 2: achieved PR Slide credit: clinicaloptions. com

Mosunetuzumab in R/R FL: Safety AE, n (%) R/R FL (N = 62) Grade 3/4 AEs in > 5% of Patients, n (%) R/R FL (N = 62) Any AE § Treatment related 60 (96. 8) 45 (72. 6) Neutropenia § Treatment-related 14 (22. 6) 10 (15. 1) Serious AE § Treatment related 22 (35. 5) 9 (14. 5) Hypophosphatemia (all treatment-related) 13 (21. 0) Grade ≥ 3 AE § Treatment related 42 (67. 7) 22 (35. 5) Anemia § Treatment related AE leading to discontinuation* § Treatment related 5 (8. 1) 4 (6. 5) Serious infections § Pneumonia § Influenza Grade 5 AE† 1 (1. 6) § *AEs leading to discontinuation: pneumonia, atrial flutter (unrelated to treatment) neutropenia, arthritis, ALT elevation (n = 1 each). †Death due to pneumonia (n = 1; onset Day 73). § Most common AEs related to therapy included hypophosphatemia, cytokine-release syndrome, and neutropenia Assouline. ASH 2020. Abstr 702. 4 (6. 5) 1 (1. 6) 12 (19. 5) 3 (4. 8) 2 (3. 2) Median time to neutropenia onset and duration: 106. 5 (1 -303) and 13 (3 -385) days; serious in 1. 6% ‒ 21/23 neutropenia cases resolved with G-CSF ‒ 21/23 events resolved by data cutoff; 1 event serious § All hypophosphatemia and anemia cases resolved; none was serious ‒ Most hypophosphatemia events resolved in cycle 1 Slide credit: clinicaloptions. com
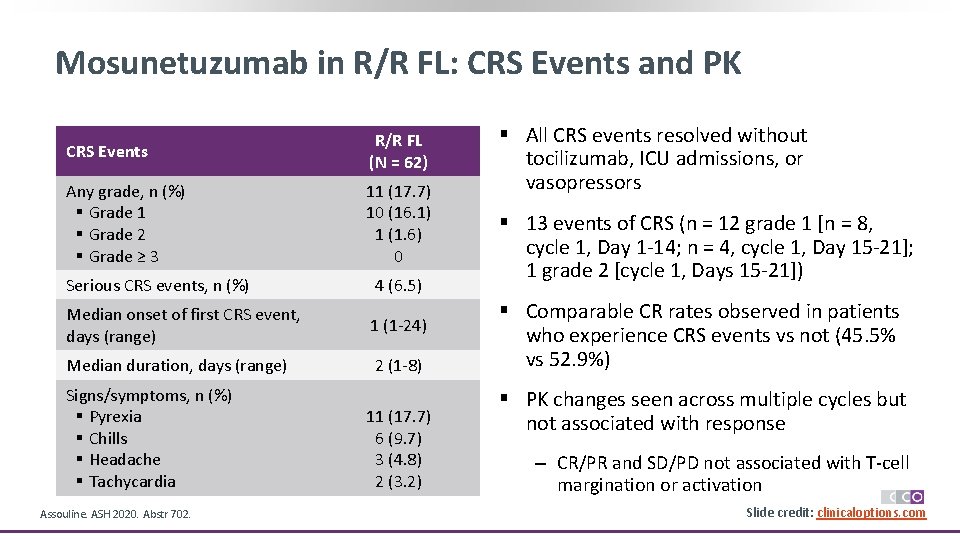
Mosunetuzumab in R/R FL: CRS Events and PK CRS Events R/R FL (N = 62) Any grade, n (%) § Grade 1 § Grade 2 § Grade ≥ 3 11 (17. 7) 10 (16. 1) 1 (1. 6) 0 Serious CRS events, n (%) 4 (6. 5) Median onset of first CRS event, days (range) 1 (1 -24) Median duration, days (range) 2 (1 -8) Signs/symptoms, n (%) § Pyrexia § Chills § Headache § Tachycardia Assouline. ASH 2020. Abstr 702. 11 (17. 7) 6 (9. 7) 3 (4. 8) 2 (3. 2) § All CRS events resolved without tocilizumab, ICU admissions, or vasopressors § 13 events of CRS (n = 12 grade 1 [n = 8, cycle 1, Day 1 -14; n = 4, cycle 1, Day 15 -21]; 1 grade 2 [cycle 1, Days 15 -21]) § Comparable CR rates observed in patients who experience CRS events vs not (45. 5% vs 52. 9%) § PK changes seen across multiple cycles but not associated with response ‒ CR/PR and SD/PD not associated with T-cell margination or activation Slide credit: clinicaloptions. com
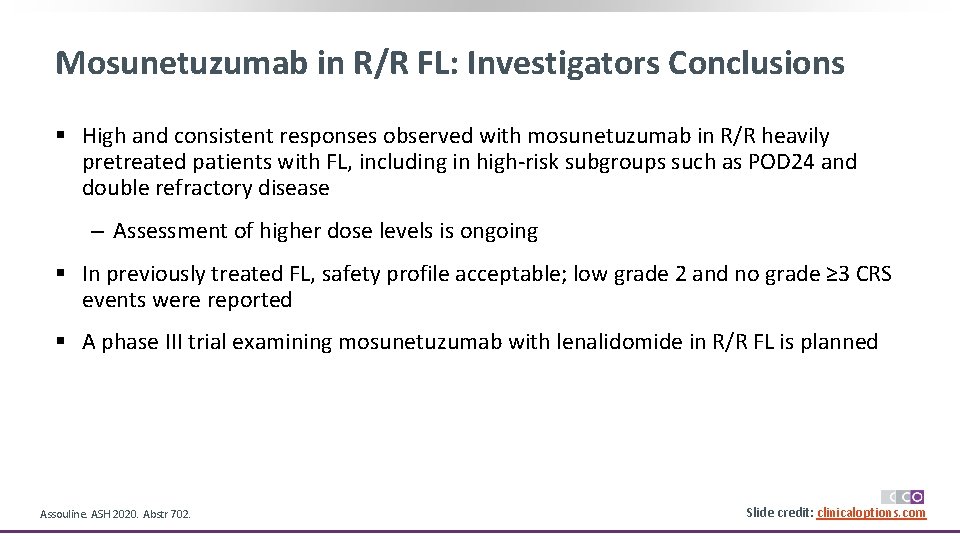
Mosunetuzumab in R/R FL: Investigators Conclusions § High and consistent responses observed with mosunetuzumab in R/R heavily pretreated patients with FL, including in high-risk subgroups such as POD 24 and double refractory disease ‒ Assessment of higher dose levels is ongoing § In previously treated FL, safety profile acceptable; low grade 2 and no grade ≥ 3 CRS events were reported § A phase III trial examining mosunetuzumab with lenalidomide in R/R FL is planned Assouline. ASH 2020. Abstr 702. Slide credit: clinicaloptions. com

Go Online for More CCO Coverage of ASH 2020! Short slideset summaries and additional CME-certified analyses with expert commentary on key studies in: § § § Acute and chronic leukemias Lymphomas and chronic lymphocytic leukemia Myelodysplastic syndromes and myeloproliferative neoplasms Myeloma Nonmalignant hematology clinicaloptions. com/oncology
- Slides: 10