Phase I Abbott Binax NOW Rapid Point of

Phase I – Abbott Binax. NOW Rapid Point of Care COVID 19 Testing for K-12 Schools October 28, 2020
Today’s presentation Department of Elementary and Secondary Education Department of Public Health/Health and Human Services • Russell Johnston • Dr. Larry Madoff • Lauren Woo • Kerin Milesky • Karen Robitaille/Caitlin Pettengill • Pam Waksmonski • Elizabeth Larsen Massachusetts Department of Elementary and Secondary Education 2

01 Rationale and Overview Protocol for Abbott Binax. NOW Testing in 02 Schools CONTENTS Required Preconditions for Onsite 03 Administration 04 Reporting Requirements 05 Next Steps and Q & A

01 Rationale and Overview

Rationale • Schools and districts across the state are working to implement the health and safety guidelines for in-person or hybrid schooling models. • To support these efforts, DESE shifted from releasing guidance to providing direct support and producing specific resources and tools. • The use of this tools and resources are increasing districts’ capacity and resiliency to keep students, faculty and staff safe, as such transmission in schools remains low. Massachusetts Department of Elementary and Secondary Education 5

Overview • The use of Abbott Binax. NOW testing in schools can rapidly identify symptomatic individuals with COVID-19 so that appropriate isolation and contact tracing can begin quickly. o 97. 1% sensitivity and 98. 5% specificity in a clinical study o Lawrence General Hospital clinical study • Test administration Massachusetts Department of Elementary and Secondary Education 6

Phase I Overview • The rollout of Phase I will help to develop standard of use in school settings. • Test kits provided at no cost. • Voluntary opportunity for schools and districts. • Potential future rollout phases will be available for districts and schools not participating in this phase. Massachusetts Department of Elementary and Secondary Education 7

02 Protocol for Abbott Binax. NOW Testing in Schools
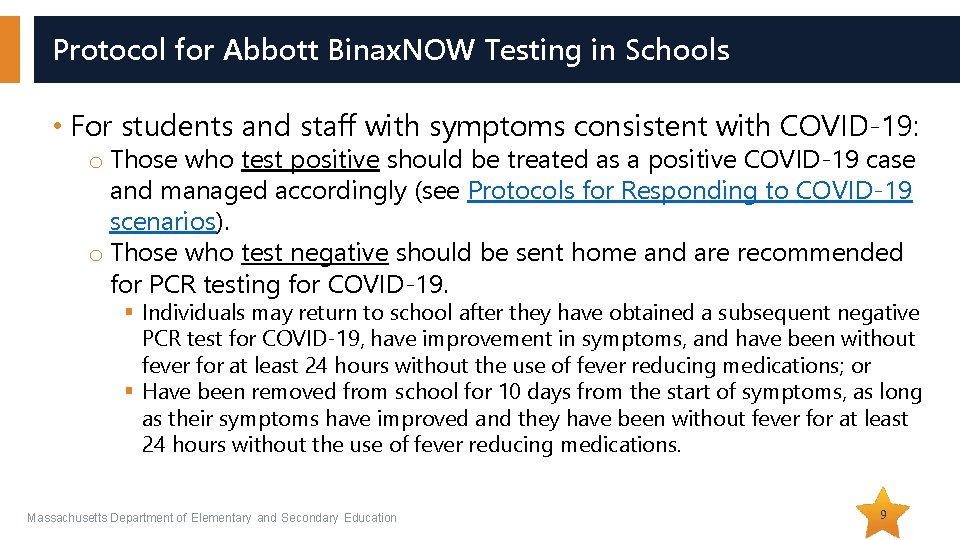
Protocol for Abbott Binax. NOW Testing in Schools • For students and staff with symptoms consistent with COVID-19: o Those who test positive should be treated as a positive COVID-19 case and managed accordingly (see Protocols for Responding to COVID-19 scenarios). o Those who test negative should be sent home and are recommended for PCR testing for COVID-19. § Individuals may return to school after they have obtained a subsequent negative PCR test for COVID-19, have improvement in symptoms, and have been without fever for at least 24 hours without the use of fever reducing medications; or § Have been removed from school for 10 days from the start of symptoms, as long as their symptoms have improved and they have been without fever for at least 24 hours without the use of fever reducing medications. Massachusetts Department of Elementary and Secondary Education 9
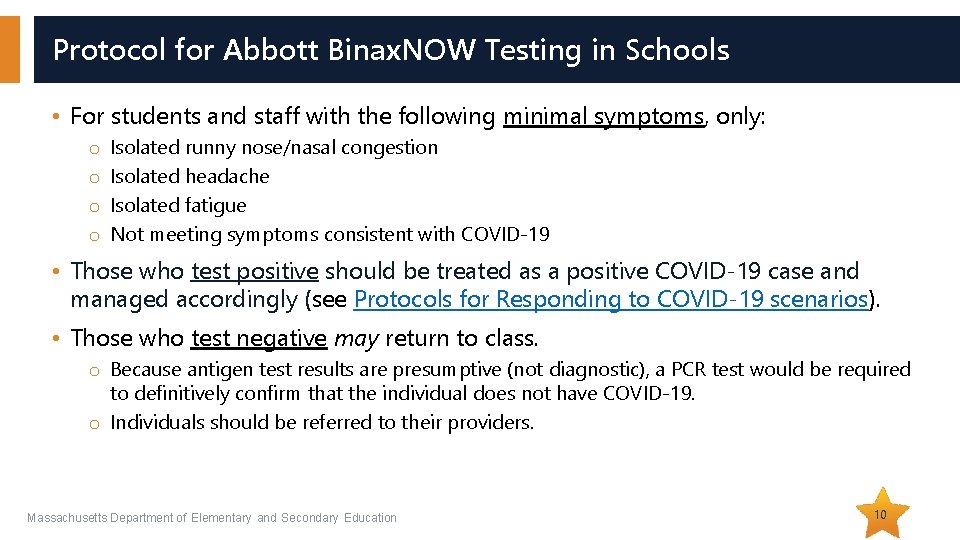
Protocol for Abbott Binax. NOW Testing in Schools • For students and staff with the following minimal symptoms, only: o o Isolated runny nose/nasal congestion Isolated headache Isolated fatigue Not meeting symptoms consistent with COVID-19 • Those who test positive should be treated as a positive COVID-19 case and managed accordingly (see Protocols for Responding to COVID-19 scenarios). • Those who test negative may return to class. o Because antigen test results are presumptive (not diagnostic), a PCR test would be required to definitively confirm that the individual does not have COVID-19. o Individuals should be referred to their providers. Massachusetts Department of Elementary and Secondary Education 10

Protocol for Abbott Binax. NOW Testing in Schools • Districts and schools must have robust and reliable ways to communicate with all families, students, teachers, and staff in order to send and receive key messages related to Abbott Binax. NOW testing. • Sample communications to families, students and staff will be provided to districts and schools participating in Phase I. Massachusetts Department of Elementary and Secondary Education 11

03 Required Preconditions for Onsite Administration

Overview of Required Preconditions • Prior to onsite administration of Abbott Binax. NOW tests, the following preconditions must be in place: o Clinical Laboratory Improvement Amendments (CLIA) Certificate of Waiver o Standing test order from physician o Appropriate personal protective equipment (PPE) o Training for school personnel o Proper authorization and consent from staff and students • Once the preconditions are met, a district or school is permitted to order the Abbott Binax. NOW tests. Massachusetts Department of Elementary and Secondary Education 13

Clinical Laboratory Improvement Amendments (CLIA) Certificate of Waiver • The Emergency Use Authorization (EUA) allows for Abbott Binax. NOW tests to be used in settings that are qualified to have the test performed and are operating under a CLIA Certificate of Waiver. • A school district may apply for one CLIA Certificate of Waiver that includes all schools in the district. • The application for a CLIA Certificate (CMS Form 116) can be found here: https: //www. cms. gov/Medicare/CMS-Forms/CMSForms/Downloads/CMS 116. pdf • For technical assistance with the application, please contact the Clinical Laboratory Program at (617) 660 -5385. Massachusetts Department of Elementary and Secondary Education 14

Standing test order from physician • The Abbott Binax. NOW test must be ordered by a physician. • Districts and schools may obtain a standing order from a school/town physician or local board of health medical director. • A model standing order will be provided to districts and schools participating in Phase I. Massachusetts Department of Elementary and Secondary Education 15

Appropriate Personal Protective Equipment (PPE) • For healthcare personnel collecting specimens or within 6 feet of individuals suspected to have COVID-19 the following PPE is required: o N 95 mask or higher-level respirator (a surgical mask can be used if a N 95 is not available) o Eye protection o Gloves o Gown, when collecting specimens Massachusetts Department of Elementary and Secondary Education 16

Training for school personnel • Health professionals, including school nurses, must complete training before administering this test. • Training videos, modules, and frequently asked questions can be found here: https: //www. globalpointofcare. abbott/en/support/productinstallation-training/navica-brand/navica-binaxnow-agtraining. html • More training options may become available soon. Massachusetts Department of Elementary and Secondary Education 17

Proper authorization and consent from staff and students • Districts and schools must obtain parent/guardian and staff authorization for administration of the Abbott Binax. NOW test. • It is recommended districts and schools proactively obtain this consent prior to administering the tests. • Three sample authorization forms will be provided to districts and schools participating in Phase I: o For students not able to consent for themselves o For students able to consent for themselves o For faculty or staff Massachusetts Department of Elementary and Secondary Education 18

Ordering the Abbott Binax. NOW tests • Once a district or school meets the five required preconditions, they may order the Abbott Binax. NOW testing kits. o DPH’s Office of Preparedness and Emergency Management will provide the test kits. • Districts and schools participating in Phase I will be given access to a specific number of test kits. o Each test kit contains 40 tests. Massachusetts Department of Elementary and Secondary Education 19

04 Reporting Requirements

Reporting requirements • Districts and schools that receive Abbott Binax. NOW test equipment must report all test results to DPH’s Bureau of Infectious Diseases and Laboratory Sciences (BIDLS) in the manner required by DPH. • A reporting mechanism will be developed for districts and schools participating in Phase I. • Districts and schools must also report positive test results to DESE’s Rapid Response Health Unit at 781 -338 -3500. Massachusetts Department of Elementary and Secondary Education 21

05 Next Steps Questions and Answers

Next Steps • By October 30, indicate initial interest in participation: https: //survey. alchemer. com/s 3/5975124/Phas e-I-Abbott-Binax. NOW-Testing-Survey • DESE will send confirmation request to school or district contact person. • DESE will send final approval to those who confirm participation. • Support for prerequisites begins immediately. Massachusetts Department of Elementary and Secondary Education 23

Additional Resources • A Rapid Test Offers Hope for Community Screening: https: //www. nytimes. com/2020/10/15/health/coronavirus-rapid-testbinaxnow. html • Rapid COVID-19 Test Shows Promise in Community Setting: https: //www. ucsf. edu/news/2020/10/418761/rapid-covid-19 -test-shows-promisecommunity-test-setting • Risk Assessment and Testing Protocols for Reducing SARS-Co. V-2 Transmission in K-12 Schools: https: //www. centerforhealthsecurity. org/ourwork/pubs_archive/pubs-pdfs/20201014 -Risk-Assessment-and-Testing. Protocols-for-Reducing-SARS-Co. V-2 -Transmission-in-K-12 -Schools. pdf Massachusetts Department of Elementary and Secondary Education

THANK YOU
- Slides: 25