PHASE EQUILIBRIA Bt2253 Chemical Thermodynamics and Bio Thermodynamics

PHASE EQUILIBRIA Bt-2253 Chemical Thermodynamics and Bio Thermodynamics Parthasarathi Subramanian, Assistant Professor, Dept. of Biotechnology, Vel Tech High Tech Engg. College. To download: www. sweetsarathi. wordpress. com
GOALS: Learn definition and basic concepts of phase equilibria and phase diagrams. Fully characterize the state of a material at a point on a phase diagram.

What is phase? � Homogeneous portion of a system � Uniform chemical and physical properties � Several phases may be present simultaneously Equilibrium (Thermodynamic Definition): � System is at equilibrium if its free energy is at a minimum. � If you change the temperature, pressure, or composition, the free energy will change.

Phase Equilibrium: Consider a closed system comprising two phases- liquid and vapor. System would be in thermal and mechanical equilibrium, another important equilibrium is phase equilibrium. Examples: Wet T-Shirt hanging in a open area dries, Aftershave � There lotion in open bottle disappears. is a driving force between the two phases, that forces the mass to transform from one phase to another. � At constant temp and pressure, when two components are mixed well, equilibrium will attain without change in Gibb’s free energy.
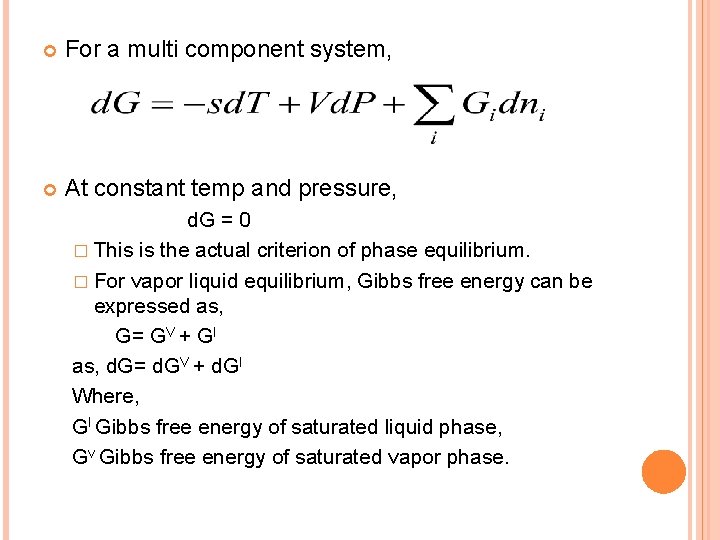
For a multi component system, At constant temp and pressure, d. G = 0 � This is the actual criterion of phase equilibrium. � For vapor liquid equilibrium, Gibbs free energy can be expressed as, G= GV + Gl as, d. G= d. GV + d. Gl Where, Gl Gibbs free energy of saturated liquid phase, Gv Gibbs free energy of saturated vapor phase.
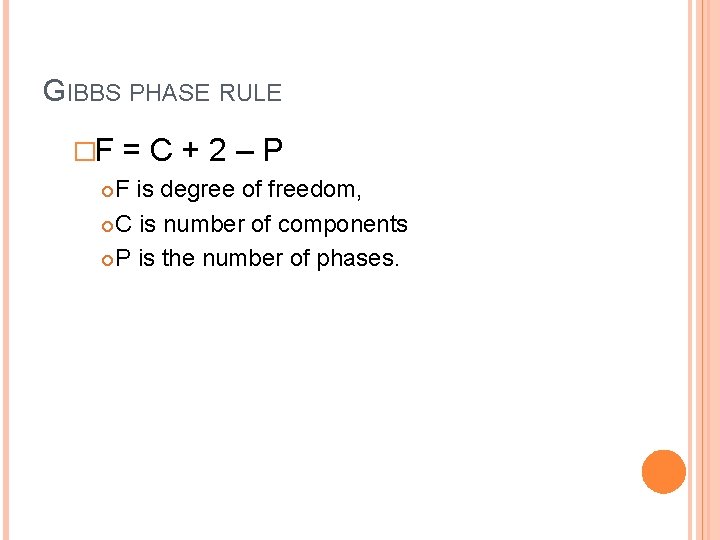
GIBBS PHASE RULE �F =C+2–P F is degree of freedom, C is number of components P is the number of phases.

For example, � Pure water boils at 100 deg, it has two phases. � Here P = 2 (Liq. and vapor phase) � C = 1 (Only water) � Then, the Gibbs phase rule, � F = C+2 -P � F = 1+2 -2 � =1 � Here the Degree of freedom is 1. It denotes the phase change occurs with the change in one variable (either temperature or pressure).

Gibbs phase rule for Ethanol-water system: �C = 2 (ethanol and water), � P = 2 (liquid and vapor), � Then, F=C+2–P = 2 + 2 – 2. = 2. � Then the degrees of freedom is 2. � For practical situations, we could consider the variable pressure is atmospheric. � Another variable could either be a temperature or the composition.

VAPOR LIQUID EQUILIBRIUM
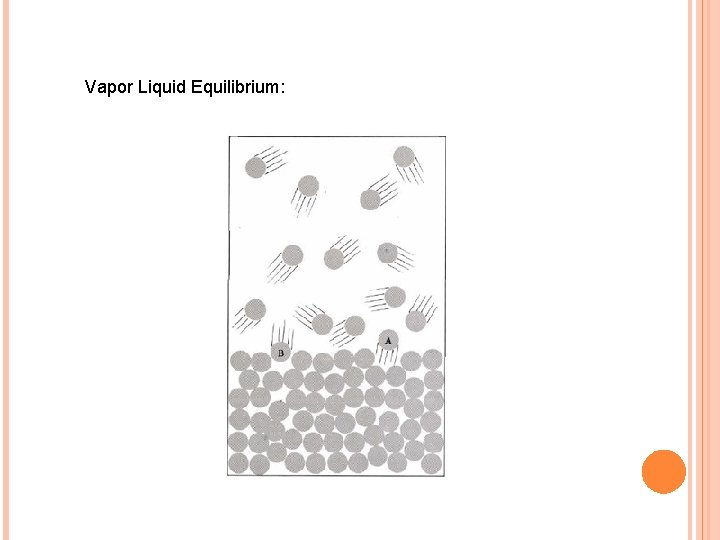
Vapor Liquid Equilibrium:
VLE of a binary system can be represented by three kinds of diagrams: � Temp-composition (T-x-y) diagrams, � Pressure-composition (P-x-y) diagrams, � Pressure-Temperature (P-T) diagrams. Temperaure - Composition (T-x-y) diagram: � Graph is plotted temperature versus composition. � Diagram is also called “Boiling point diagram”. � We can understand how the equilibrium curve changes with the diagram.
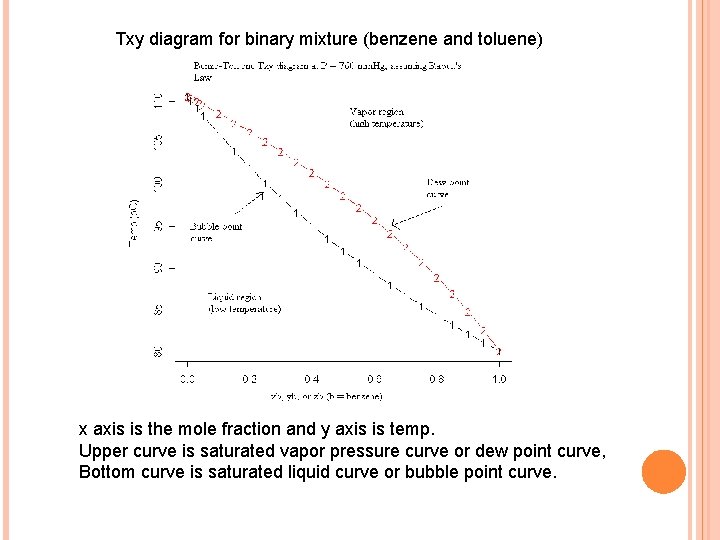
Txy diagram for binary mixture (benzene and toluene) x axis is the mole fraction and y axis is temp. Upper curve is saturated vapor pressure curve or dew point curve, Bottom curve is saturated liquid curve or bubble point curve.

� Region bound by two curves is two phase region (partially liquid and partially vapor). � At any point below the lower curve is liquid completely and vise versa. � Region below bubble point curve is sub-cooled region (sub cooled liquid)and above dew point curve is super heated region (super heated vapor).
- Slides: 13