PHASE DIAGRAMS PHASE CHANGE DIAGRAM WHAT IS A








- Slides: 11

PHASE DIAGRAMS

PHASE CHANGE DIAGRAM

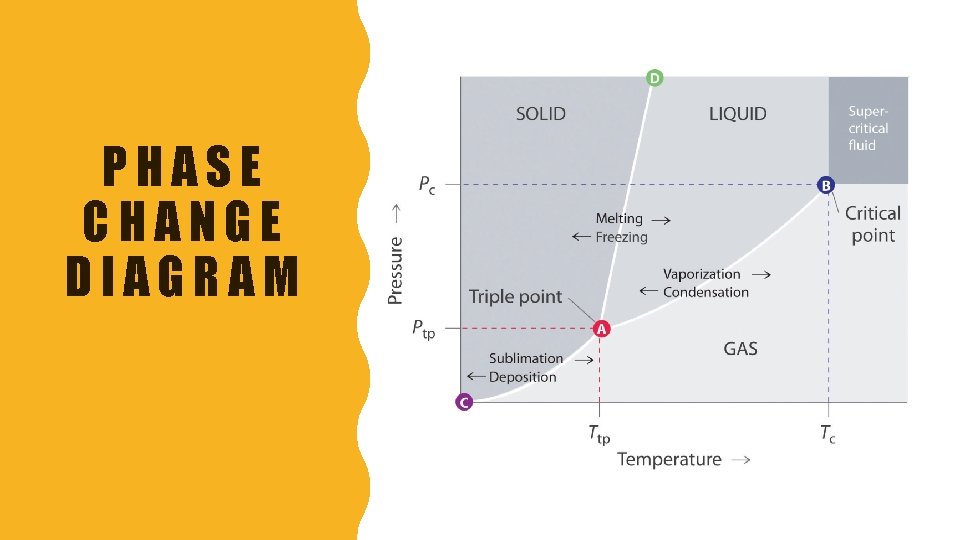
WHAT IS A PHASE DIAGRAM? • Graphical representation of the physical states of a substance under different conditions of Temperature and Pressure • Typically: – Pressure = y-axis – Temperature = x-axis • As we cross the lines/curve, a phase change occurs • On the line, two states of the substance coexists in equilibrium on the lines or curves

WHAT IS A TRIPLE POINT? • The point on a phase diagram at which the 3 states of matter: gas, liquid, and solid coexist

CYCLOHEXANE AT TRIPLE POINT

WHAT IS THE CRITICAL POINT? • The point on a phase diagram where the substance is indistinguishable between liquid and gaseous states –Called supercritical fluids

IS SUPERCRITICAL FLUID THE SAME AS PLASMA? • No! • Supercritical fluid = properties of liquid and gas • Plasma = ionized media or phase (where electrons are separated because of high pressure and/or higher temperature to form positively charged atoms and free electrons)

FYI… • With most substances, the temperature and pressure related to the triple point lie below standard temperature and pressure and the pressure for the critical point lies above standard pressure • Therefore, at standard pressure as temp increases, most substances change from solid to liquid to gas, and at standard temp, as pressure increases, most substances change from gas to liquid to solid

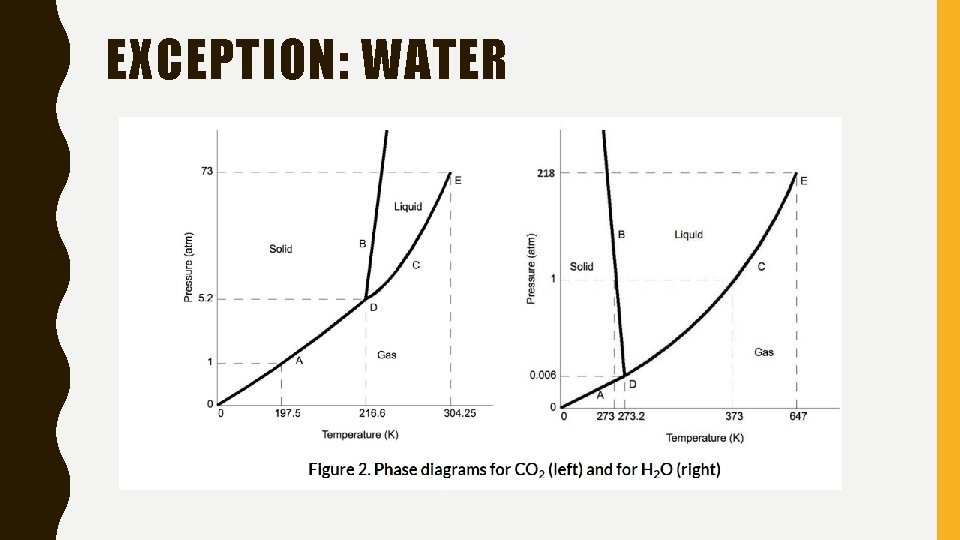
EXCEPTION: WATER

LOOKING AT THE SLOPES • Normally: Solid/Liquid phase line slopes positively to the right • Water: slopes to the left

WHY? ? • Shows that the liquid phase is more dense than the solid phase – Because of crystal structure of the solid phase • For water, the molecules crystalize in a lattice with greater average space between the molecules – Results in a solid with a lower density than the liquid • Reason why we can melt ice simply by applying pressure and not by adding heat