Phase Diagrams melting production process alloying strength Tm

- Slides: 20


Phase Diagrams melting / production process / alloying (strength, Tm. . . ) heat treatment material properties microstructure system (e. g. Cu-Ni) components (pure substances or compounds) phases (uniform physical/chemical characteristic) solid solution – solvent + solute solubility limit=> precipitation pure materials – 2 phases: liquid + solid

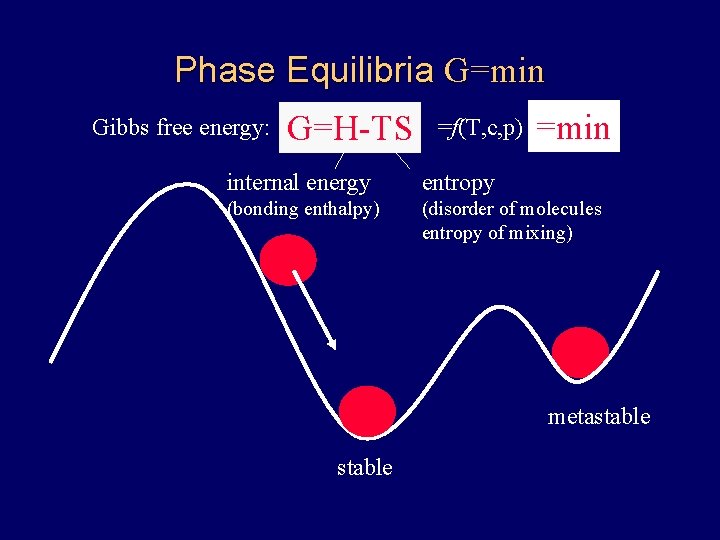
Phase Equilibria G=min Gibbs free energy: G=H-TS =f(T, c, p) =min internal energy entropy (bonding enthalpy) (disorder of molecules entropy of mixing) metastable

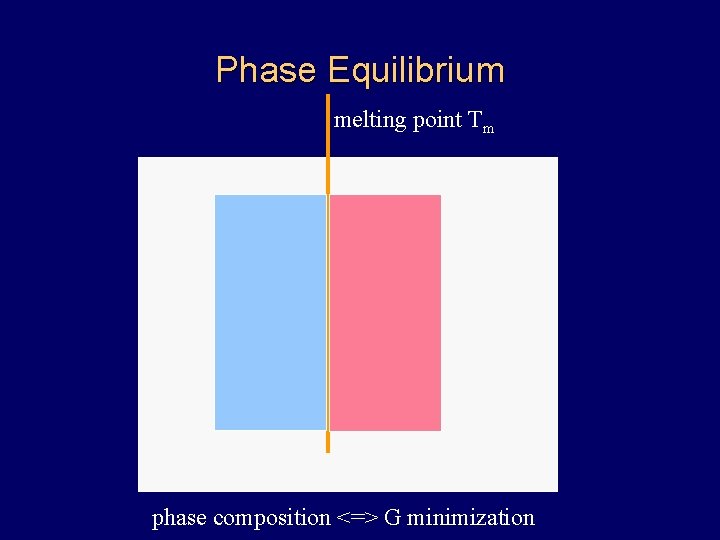
Phase Equilibrium melting point Tm phase composition <=> G minimization

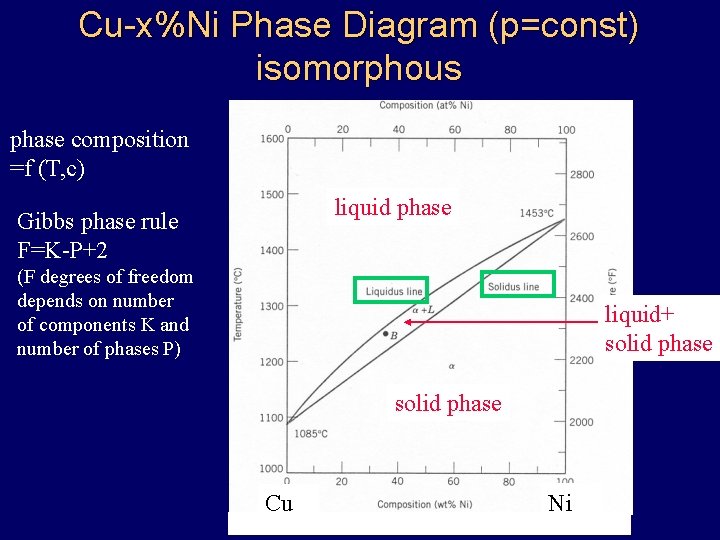
Cu-x%Ni Phase Diagram (p=const) isomorphous phase composition =f (T, c) liquid phase Gibbs phase rule F=K-P+2 (F degrees of freedom depends on number of components K and number of phases P) liquid+ solid phase Cu Ni

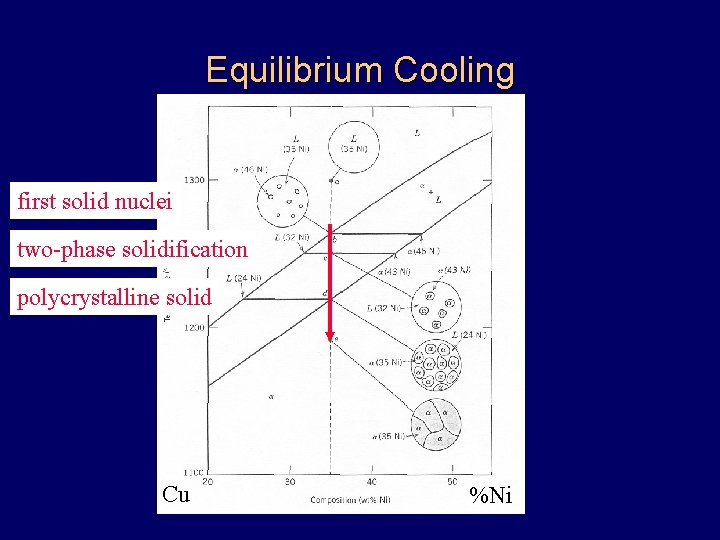
Equilibrium Cooling first solid nuclei two-phase solidification polycrystalline solid Cu %Ni

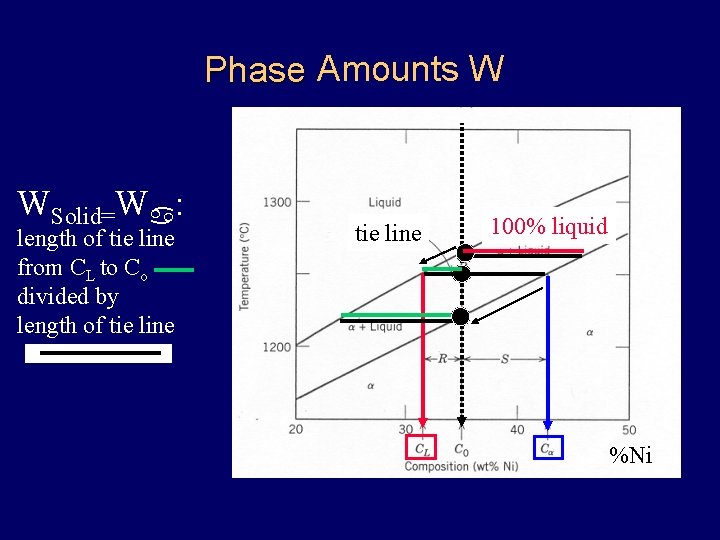
Amounts W Phase Composition WSolid=Wa: length of tie line from CL to Co divided by length of tie line 100% liquid %Ni

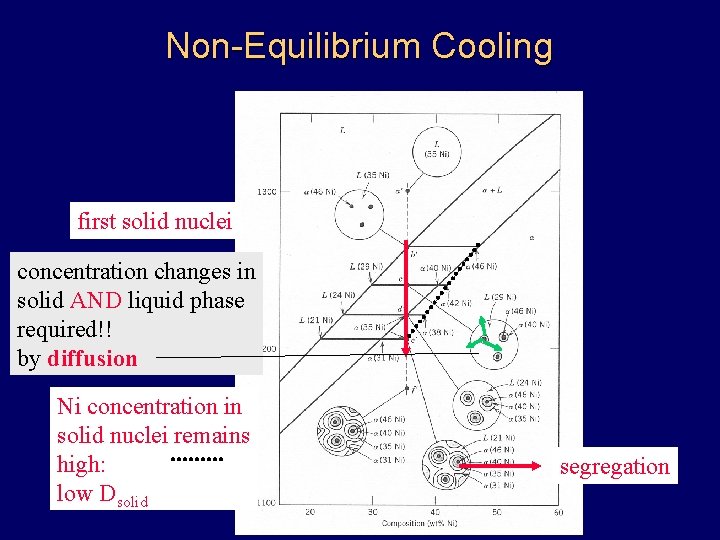
Non-Equilibrium Cooling first solid nuclei concentration changes in solid AND liquid phase required!! by diffusion Ni concentration in solid nuclei remains high: low Dsolid segregation

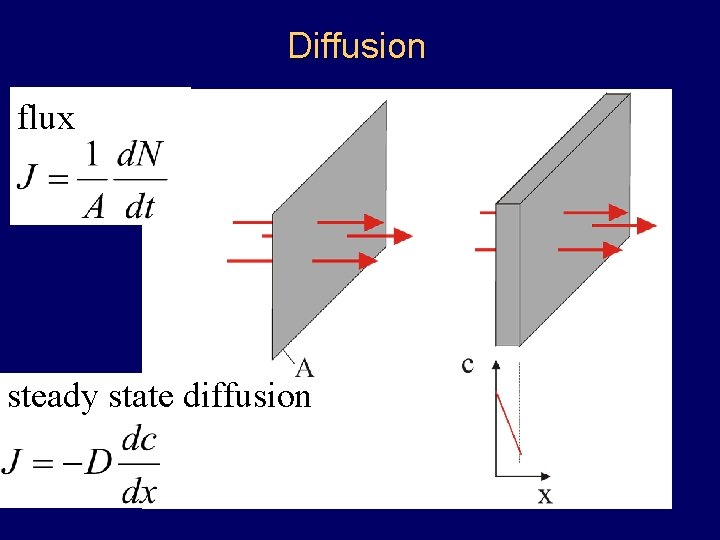
Diffusion flux steady state diffusion

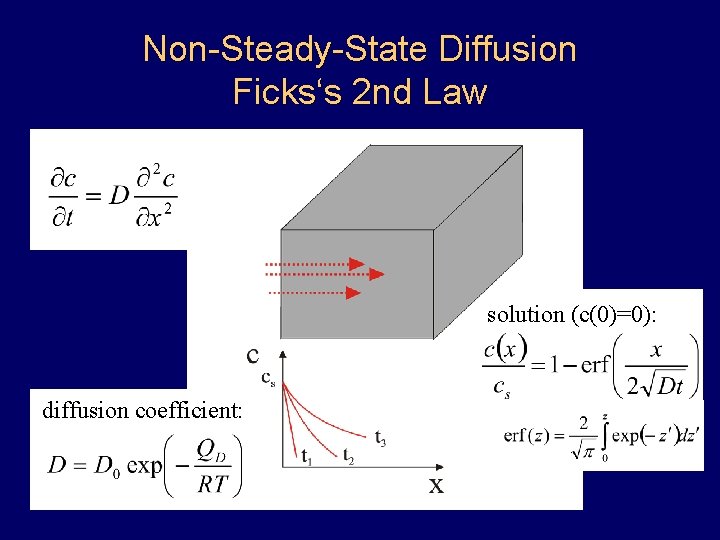
Non-Steady-State Diffusion Ficks‘s 2 nd Law solution (c(0)=0): diffusion coefficient:

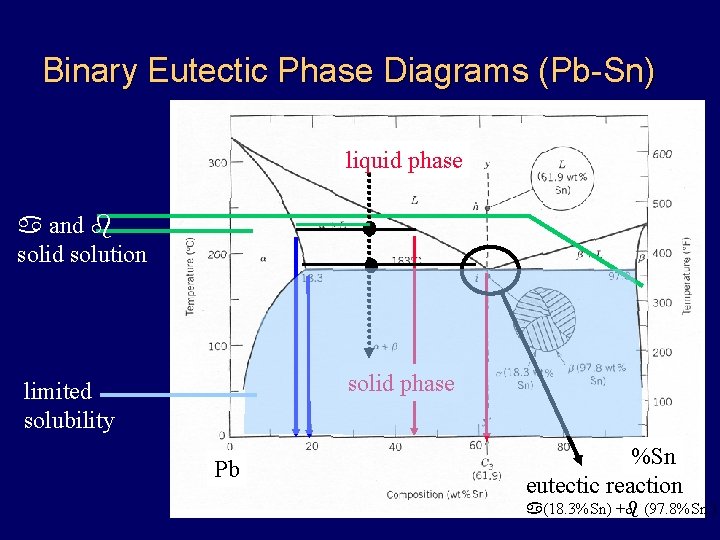
Binary Eutectic Phase Diagrams (Pb-Sn) liquid phase a and b solid solution solid phase limited solubility Pb %Sn eutectic reaction a(18. 3%Sn) +b (97. 8%Sn )

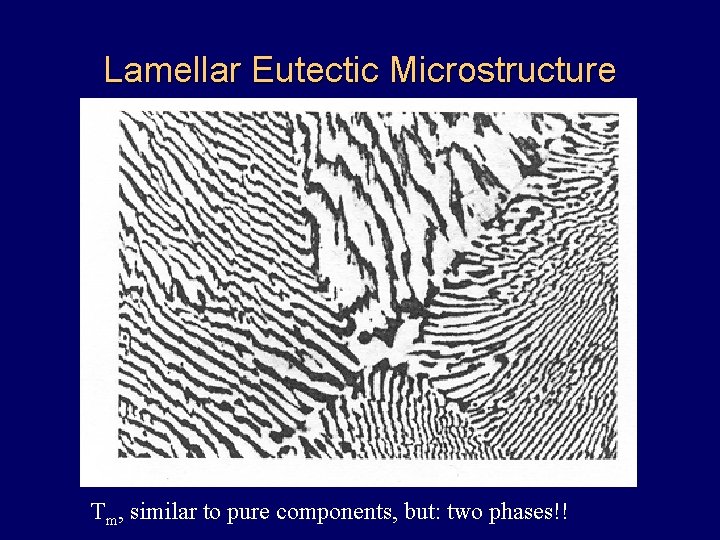
Lamellar Eutectic Microstructure Tm, similar to pure components, but: two phases!!

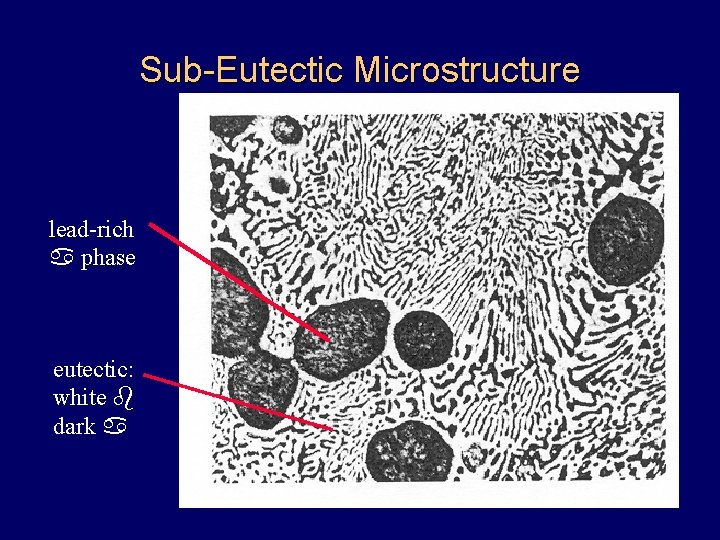
Sub-Eutectic Microstructure lead-rich a phase eutectic: white b dark a

Microstructural Examination Metallography preparation of cross section -cutting -grinding (Si. C paper) -polishing (e. g. 1µm diamond suspension) -etching (selective chemical attack of grains/grain boundaries) optical microscopy electron microscopy (transmission (TEM) or scanning (SEM))

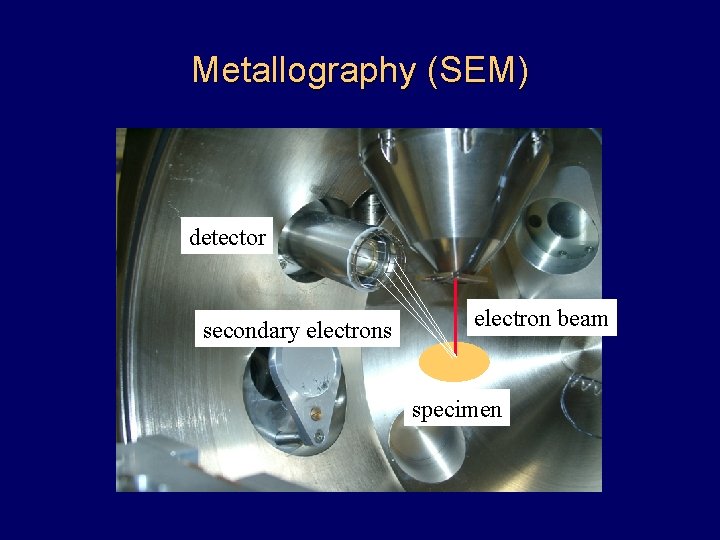
Metallography (SEM) detector secondary electrons electron beam specimen

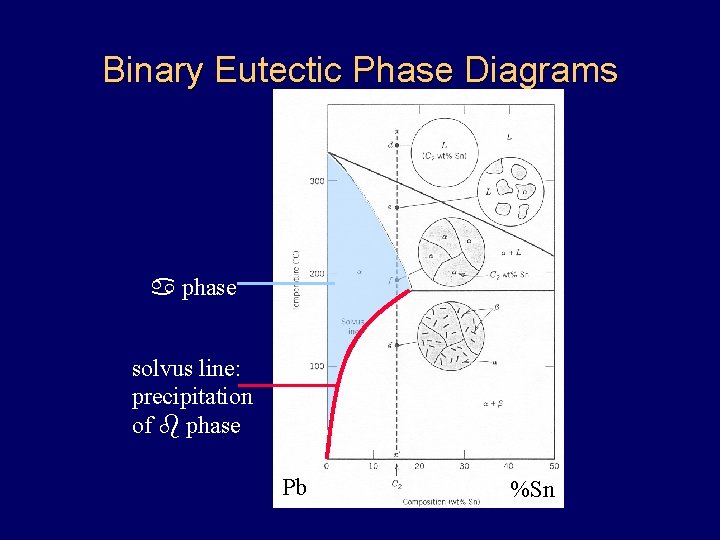
Binary Eutectic Phase Diagrams a phase solvus line: precipitation of b phase Pb %Sn

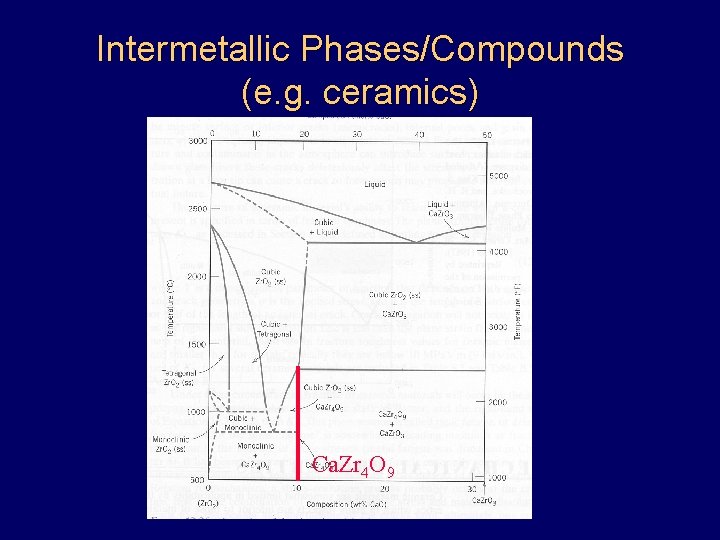
Intermetallic Phases/Compounds (e. g. ceramics) Ca. Zr 4 O 9

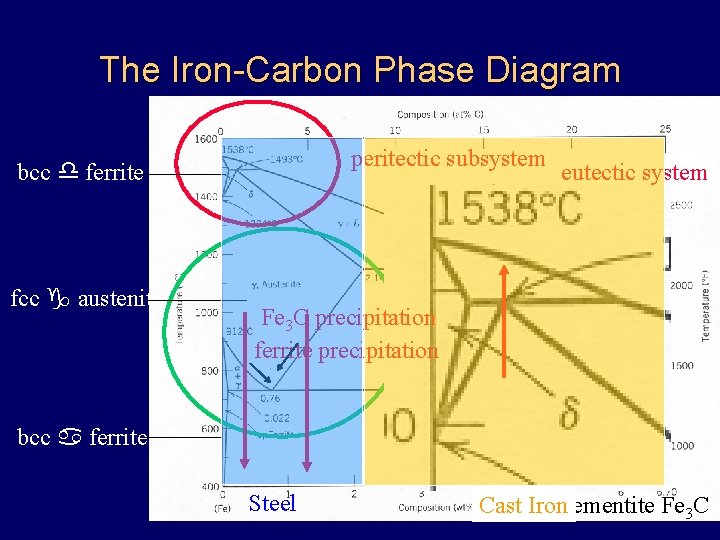
The Iron-Carbon Phase Diagram peritectic subsystem bcc d ferrite fcc g austenite eutectic system Fe 3 C precipitation eutectoid subsystem ferrite precipitation bcc a ferrite Steel Cast Ironcementite Fe 3 C

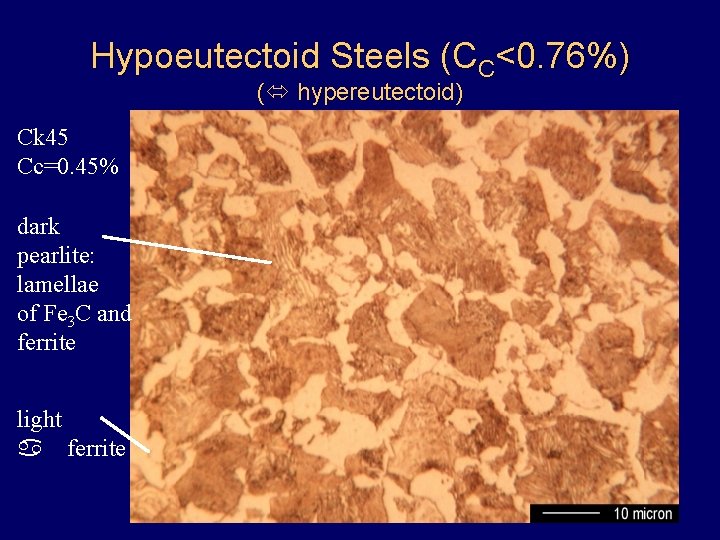
Hypoeutectoid Steels (CC<0. 76%) ( hypereutectoid) Ck 45 Cc=0. 45% dark pearlite: lamellae of Fe 3 C and ferrite light a ferrite

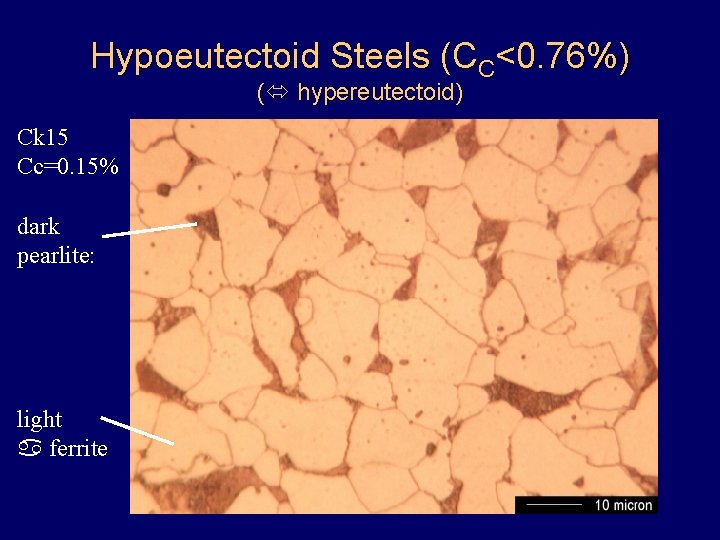
Hypoeutectoid Steels (CC<0. 76%) ( hypereutectoid) Ck 15 Cc=0. 15% dark pearlite: light a ferrite