Phase Diagrams and the Equilibrium of Substances 1
Phase Diagrams and the Equilibrium of Substances 1
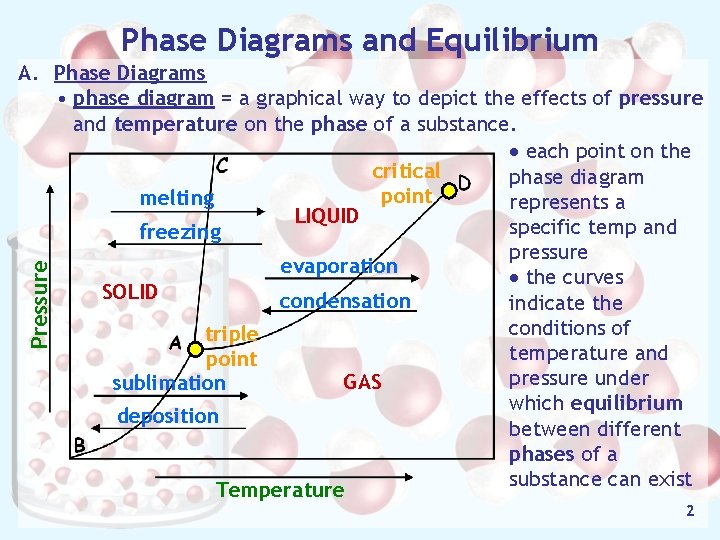
Phase Diagrams and Equilibrium Pressure A. Phase Diagrams • phase diagram = a graphical way to depict the effects of pressure and temperature on the phase of a substance. · each point on the critical phase diagram point melting represents a LIQUID specific temp and freezing pressure evaporation · the curves SOLID condensation indicate the conditions of triple temperature and point pressure under GAS sublimation which equilibrium deposition between different phases of a substance can exist Temperature 2
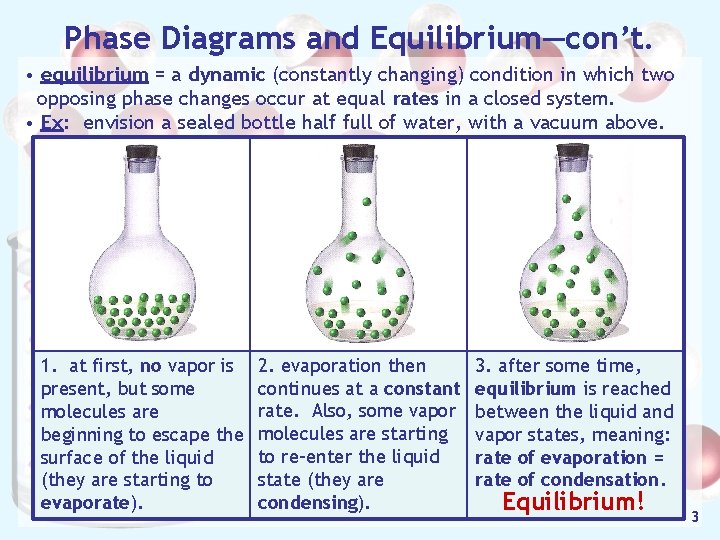
Phase Diagrams and Equilibrium—con’t. • equilibrium = a dynamic (constantly changing) condition in which two opposing phase changes occur at equal rates in a closed system. • Ex: envision a sealed bottle half full of water, with a vacuum above. 1. at first, no vapor is present, but some molecules are beginning to escape the surface of the liquid (they are starting to evaporate). 2. evaporation then continues at a constant rate. Also, some vapor molecules are starting to re-enter the liquid state (they are condensing). 3. after some time, equilibrium is reached between the liquid and vapor states, meaning: rate of evaporation = rate of condensation. Equilibrium! 3
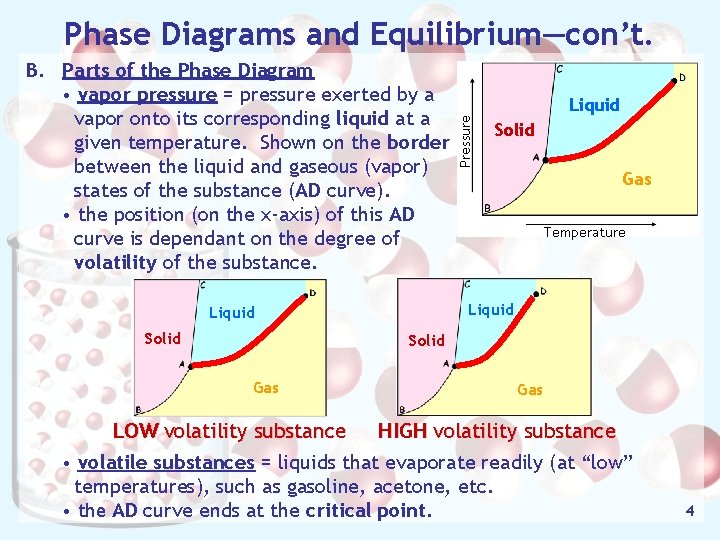
Phase Diagrams and Equilibrium—con’t. Solid Gas Temperature Liquid Solid Liquid Pressure B. Parts of the Phase Diagram • vapor pressure = pressure exerted by a vapor onto its corresponding liquid at a given temperature. Shown on the border between the liquid and gaseous (vapor) states of the substance (AD curve). • the position (on the x-axis) of this AD curve is dependant on the degree of volatility of the substance. Solid Gas LOW volatility substance HIGH volatility substance • volatile substances = liquids that evaporate readily (at “low” temperatures), such as gasoline, acetone, etc. • the AD curve ends at the critical point. 4
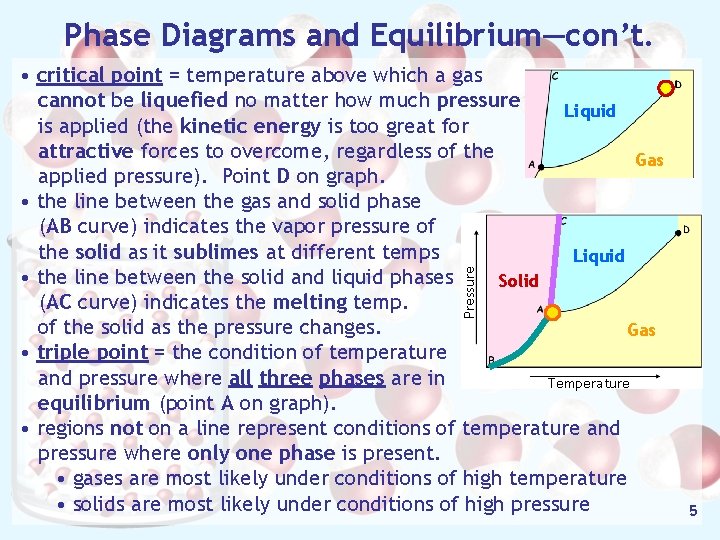
Phase Diagrams and Equilibrium—con’t. Pressure • critical point = temperature above which a gas cannot be liquefied no matter how much pressure Liquid is applied (the kinetic energy is too great for attractive forces to overcome, regardless of the Gas applied pressure). Point D on graph. • the line between the gas and solid phase (AB curve) indicates the vapor pressure of the solid as it sublimes at different temps Liquid • the line between the solid and liquid phases Solid (AC curve) indicates the melting temp. of the solid as the pressure changes. Gas • triple point = the condition of temperature and pressure where all three phases are in Temperature equilibrium (point A on graph). • regions not on a line represent conditions of temperature and pressure where only one phase is present. • gases are most likely under conditions of high temperature • solids are most likely under conditions of high pressure 5
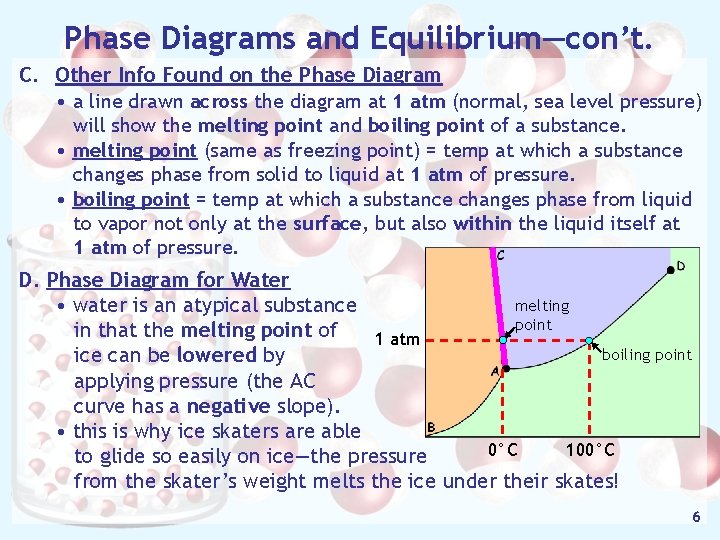
Phase Diagrams and Equilibrium—con’t. C. Other Info Found on the Phase Diagram • a line drawn across the diagram at 1 atm (normal, sea level pressure) will show the melting point and boiling point of a substance. • melting point (same as freezing point) = temp at which a substance changes phase from solid to liquid at 1 atm of pressure. • boiling point = temp at which a substance changes phase from liquid to vapor not only at the surface, but also within the liquid itself at 1 atm of pressure. D. Phase Diagram for Water melting • water is an atypical substance point in that the melting point of 1 atm boiling point ice can be lowered by applying pressure (the AC curve has a negative slope). • this is why ice skaters are able 0°C 100°C to glide so easily on ice—the pressure from the skater’s weight melts the ice under their skates! 6
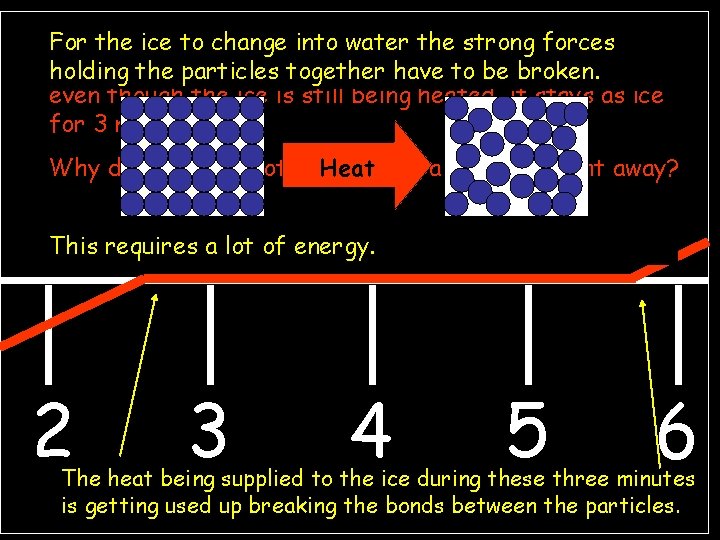
Temperature (°C) howinto thewater temperature of ice changes For Graph the icetotoshow change Is the it astrong solid, forces as together itisisagently heated This part ofparticles the graph flatliquid line. Itbe shows that holding the have to broken. or gas? even 100 though the ice is still being heated, A gas it stays as ice for 90 3 minutes. 80 Why does the ice not change Heat into a liquid straight away? 70 60 This 50 40 Is it a solid, requires a liquid lot of. A energy. liquid or gas? 30 20 10 0 2 -10 -20 1 2 3 3 4 5 6 4 7 8 5 6 9 10 11 12 13 14 15 16 solid The heat A being supplied to the ice during these three minutes Is it a Time solid, (minutes) is getting usedliquid up breaking the bonds between the particles. or gas?
- Slides: 7