Phase Diagrams 1 2 TRANSITIONS BETWEEN PHASES Section
Phase Diagrams 1
2 TRANSITIONS BETWEEN PHASES Section 13. 10 Lines connect all conditions of T and P where EQUILIBRIUM exists between the phases on either side of the line. (At equilibrium particles move from liquid to gas as fast as they move from gas to liquid, for example. )
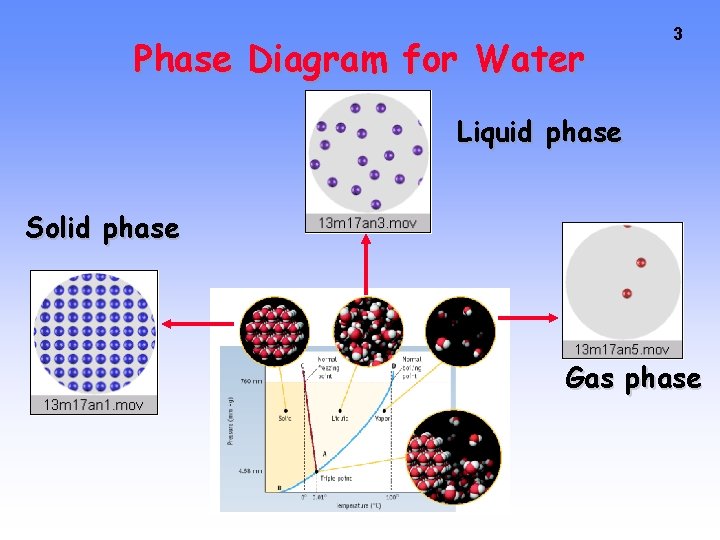
Phase Diagram for Water 3 Liquid phase Solid phase Gas phase
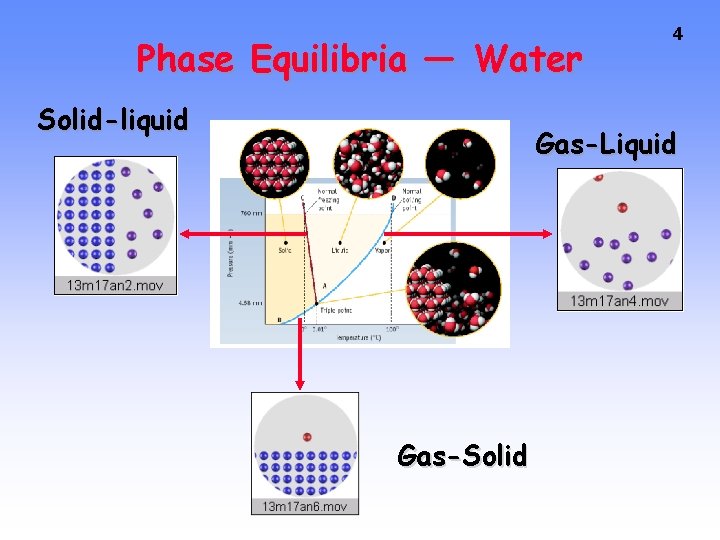
Phase Equilibria — Water Solid-liquid 4 Gas-Liquid Gas-Solid
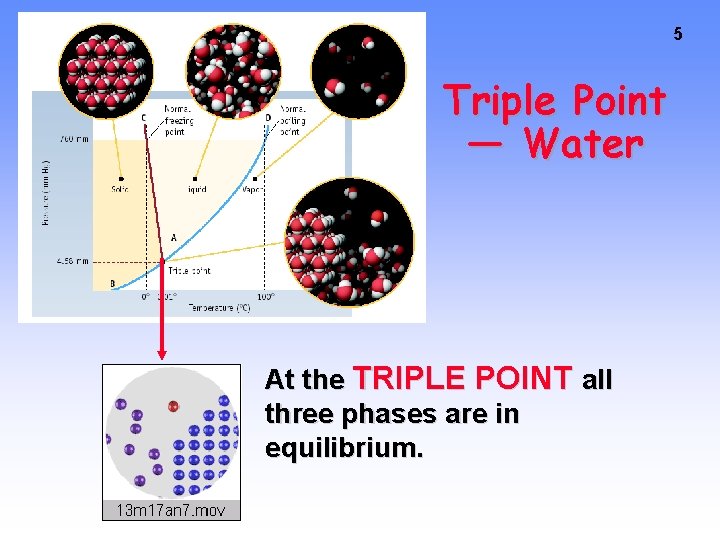
5 Triple Point — Water At the TRIPLE POINT all three phases are in equilibrium.
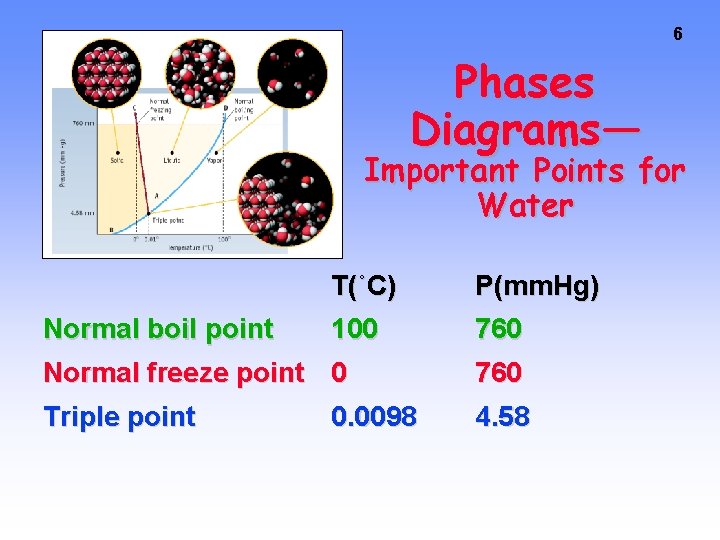
6 Phases Diagrams— Important Points for Water Normal boil point T(˚C) P(mm. Hg) 100 760 Normal freeze point 0 760 Triple point 4. 58 0. 0098

7 Critical T and P As P and T increase, you finally reach the CRITICAL T and P Above critical T no liquid exists no matter how high the pressure.
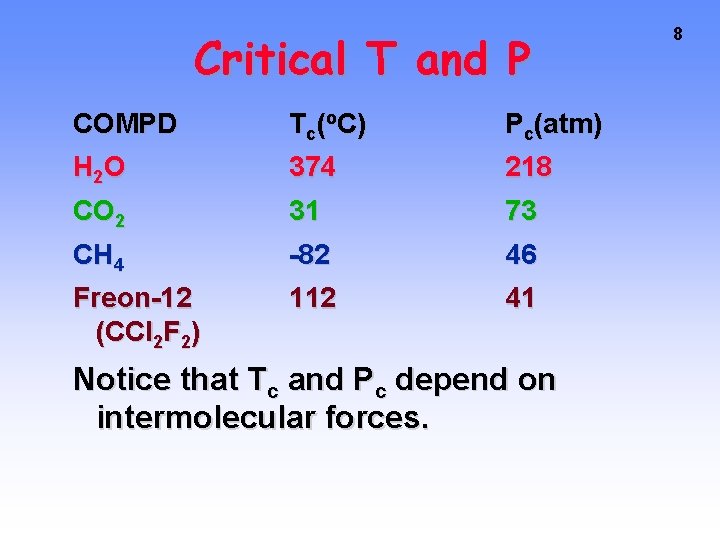
Critical T and P COMPD T c (o C ) Pc(atm) H 2 O 374 218 CO 2 31 73 CH 4 -82 46 Freon-12 (CCl 2 F 2) 112 41 Notice that Tc and Pc depend on intermolecular forces. 8
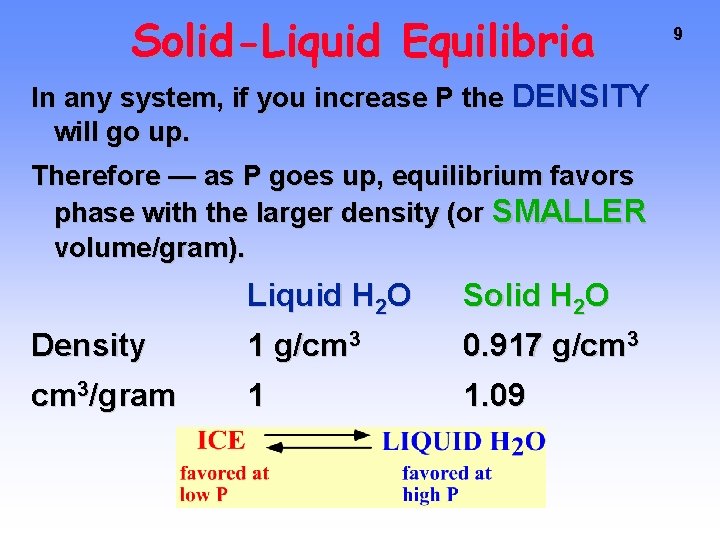
Solid-Liquid Equilibria In any system, if you increase P the DENSITY will go up. Therefore — as P goes up, equilibrium favors phase with the larger density (or SMALLER volume/gram). Liquid H 2 O Solid H 2 O Density 1 g/cm 3 0. 917 g/cm 3/gram 1 1. 09 9
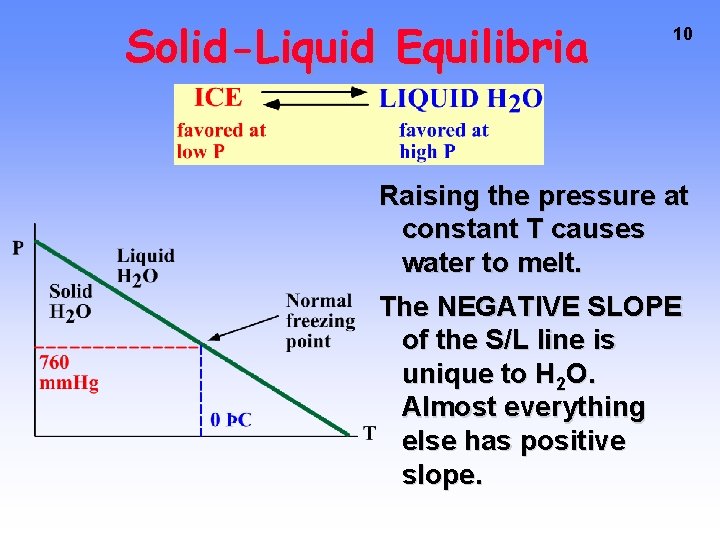
Solid-Liquid Equilibria 10 Raising the pressure at constant T causes water to melt. The NEGATIVE SLOPE of the S/L line is unique to H 2 O. Almost everything else has positive slope.
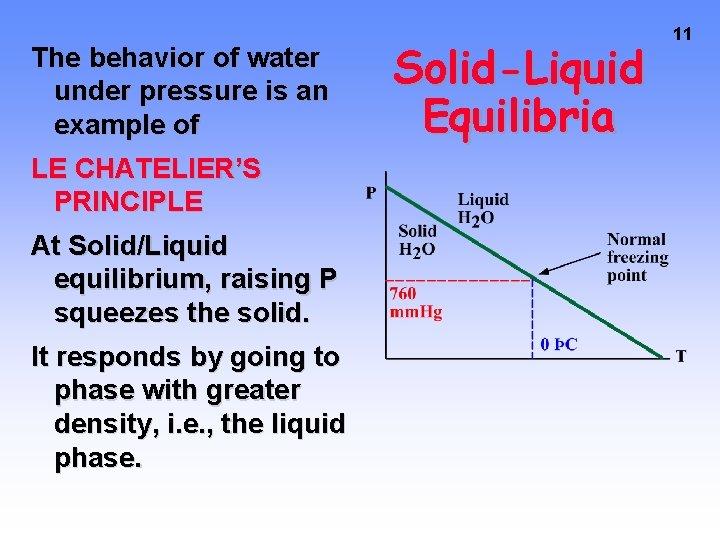
The behavior of water under pressure is an example of LE CHATELIER’S PRINCIPLE At Solid/Liquid equilibrium, raising P squeezes the solid. It responds by going to phase with greater density, i. e. , the liquid phase. Solid-Liquid Equilibria 11

12 Solid-Vapor Equilibria At P < 4. 58 mm. Hg and T < 0. 0098 ˚C solid H 2 O can go directly to vapor. This process is called SUBLIMATION This is how a frost-free refrigerator works.
- Slides: 12