Phase diagram of immiscible liquidliquid binary systems F2
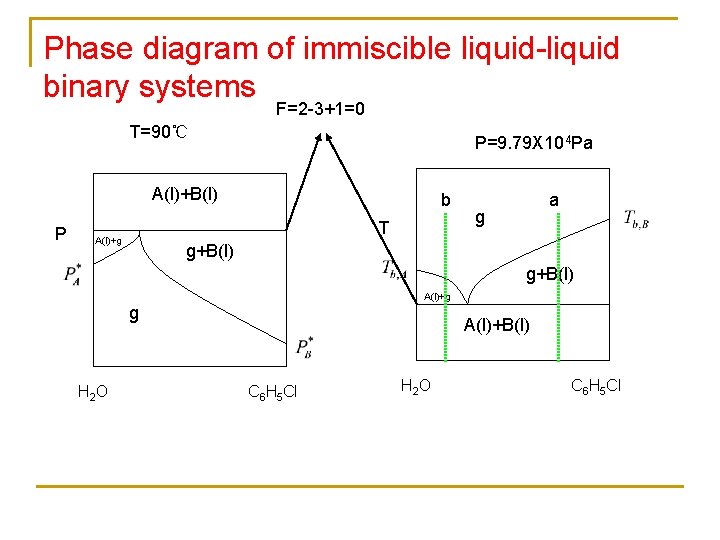
Phase diagram of immiscible liquid-liquid binary systems F=2 -3+1=0 T=90℃ P=9. 79 X 104 Pa A(l)+B(l) P b T A(l)+g a g g+B(l) A(l)+g g H 2 O A(l)+B(l) C 6 H 5 Cl H 2 O C 6 H 5 Cl
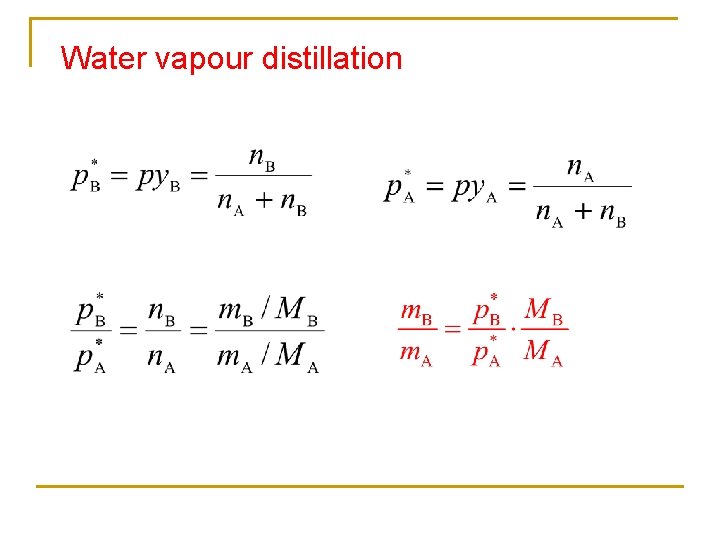
Water vapour distillation
5. 4. 4 Phase diagrams of solid-liquid binary systems n Condensed matter Solid-solution n Water-salt n ( )P F=2 -Φ+1 n n Single phase region Bi-phase line Triple point
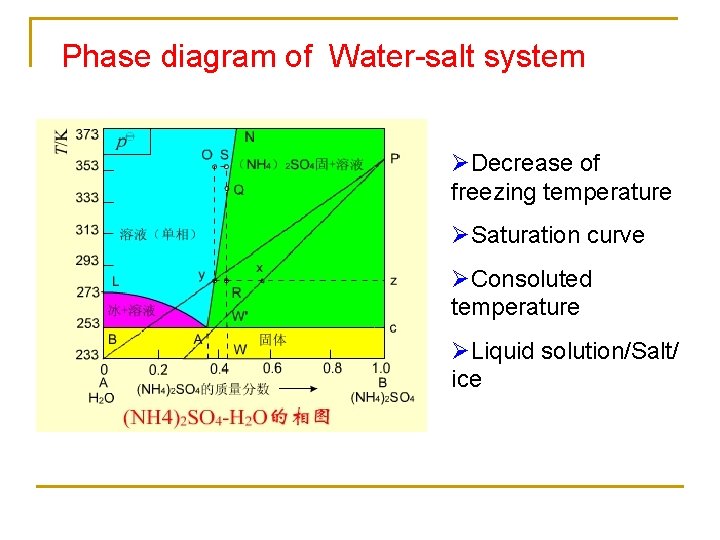
Phase diagram of Water-salt system ØDecrease of freezing temperature ØSaturation curve ØConsoluted temperature ØLiquid solution/Salt/ ice
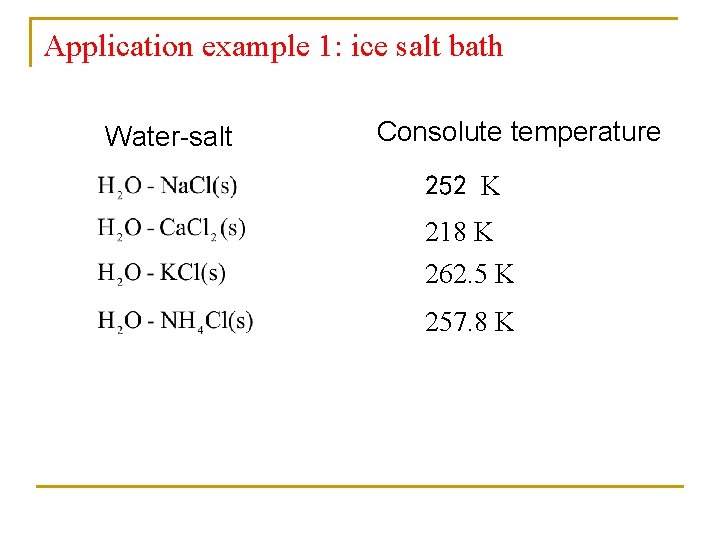
Application example 1: ice salt bath Water-salt Consolute temperature 252 K 218 K 262. 5 K 257. 8 K
Application example 2: Refine crude salt
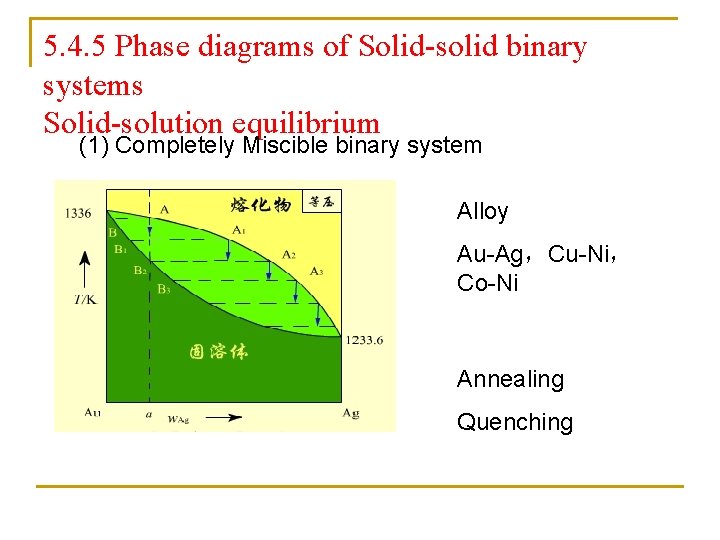
5. 4. 5 Phase diagrams of Solid-solid binary systems Solid-solution equilibrium (1) Completely Miscible binary system Alloy Au-Ag,Cu-Ni, Co-Ni Annealing Quenching
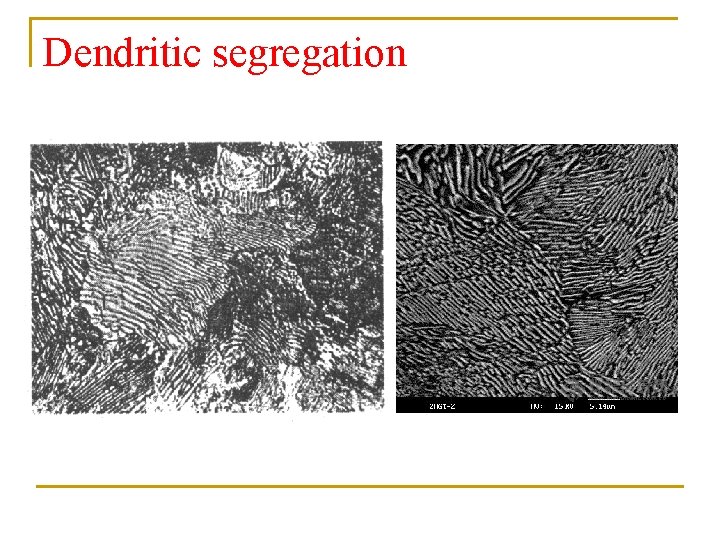
Dendritic segregation
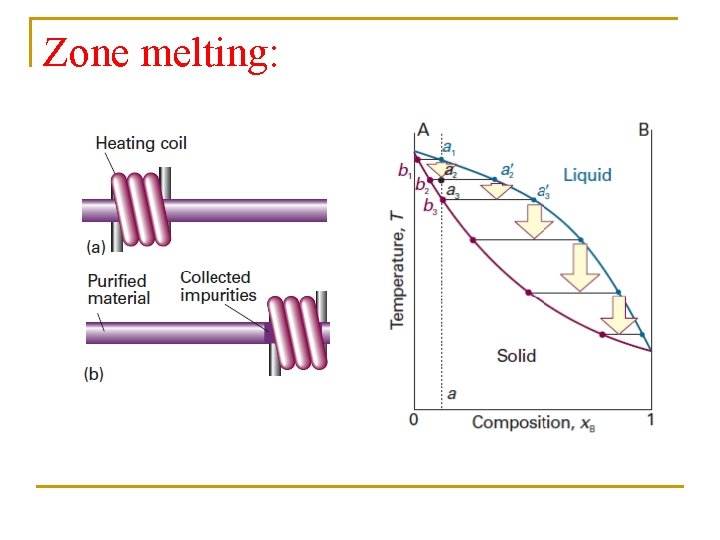
Zone melting:
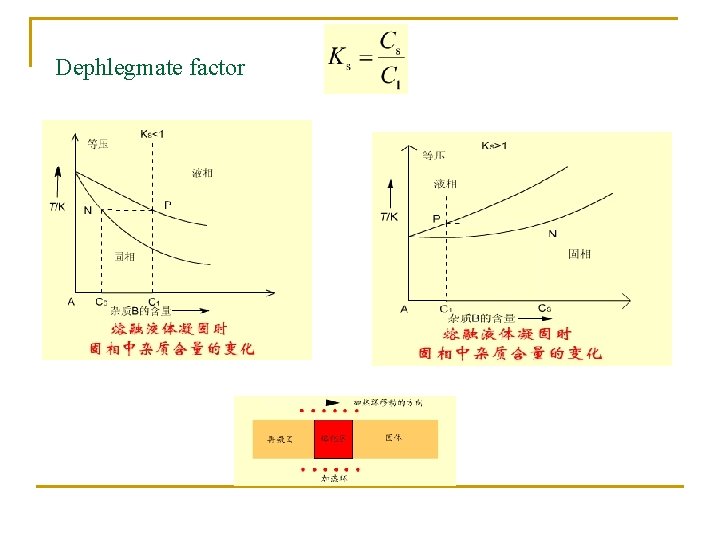
Dephlegmate factor
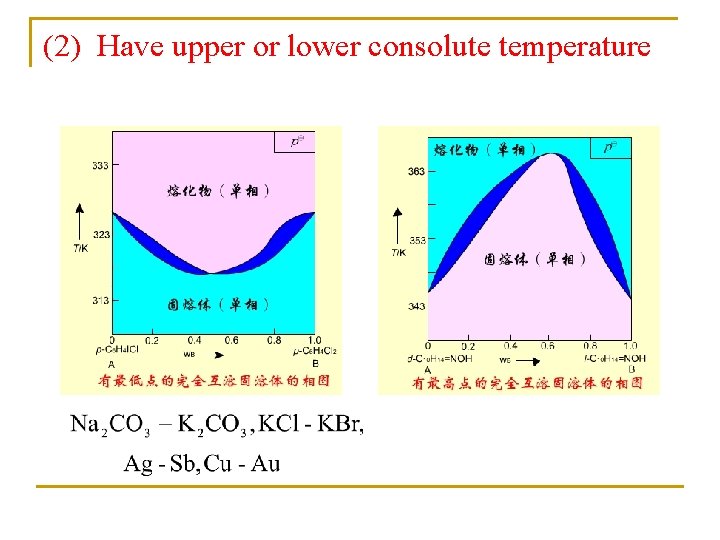
(2) Have upper or lower consolute temperature
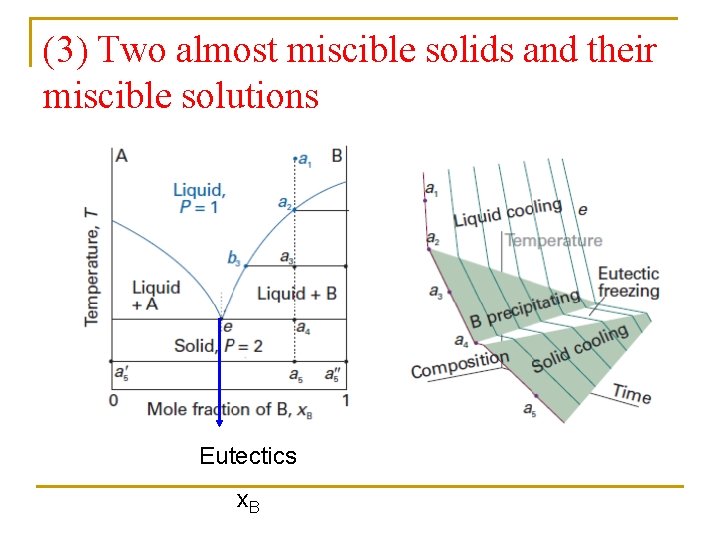
(3) Two almost miscible solids and their miscible solutions Eutectics x. B
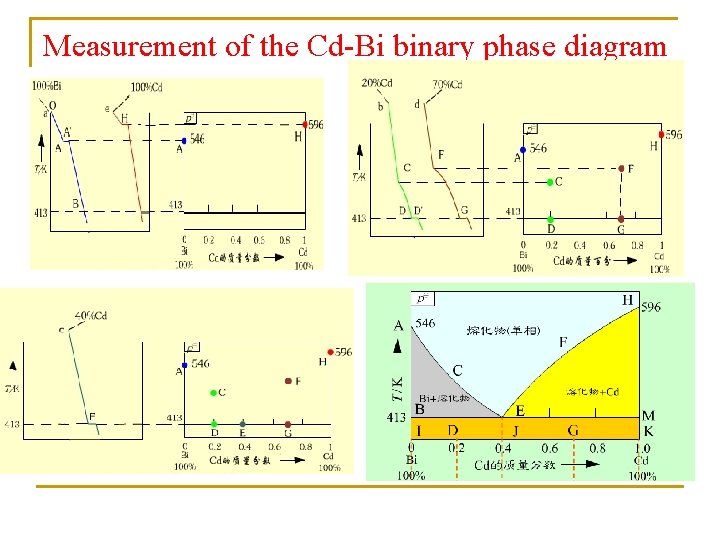
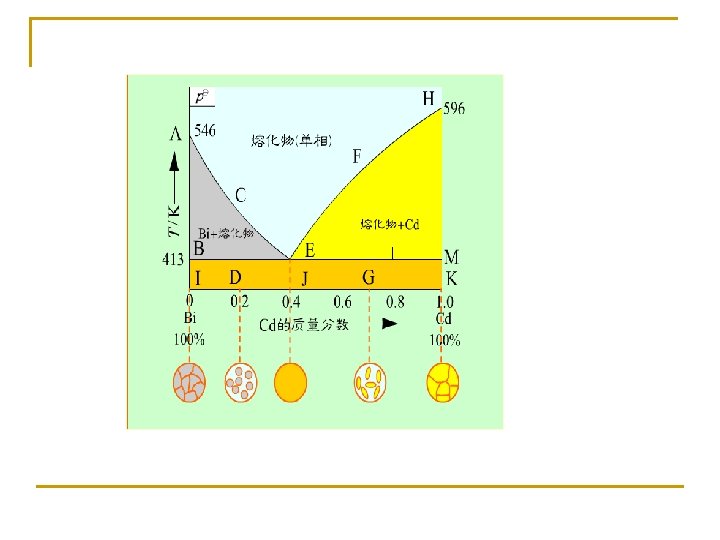
Measurement of the Cd-Bi binary phase diagram
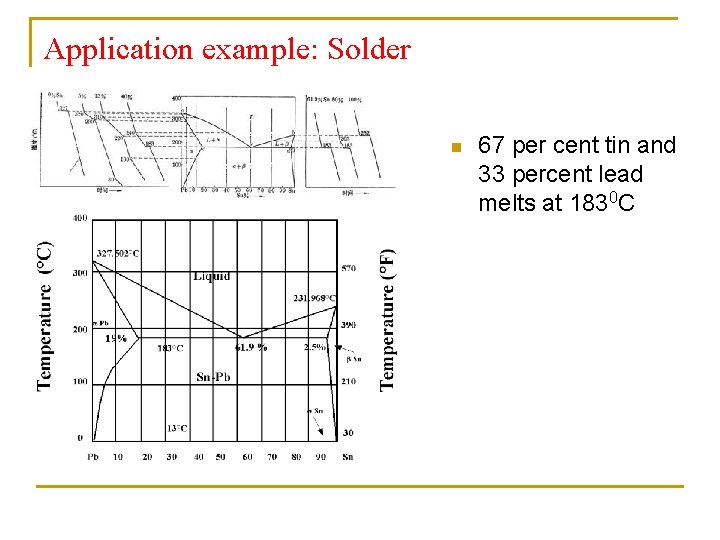
Application example: Solder n 67 per cent tin and 33 percent lead melts at 1830 C
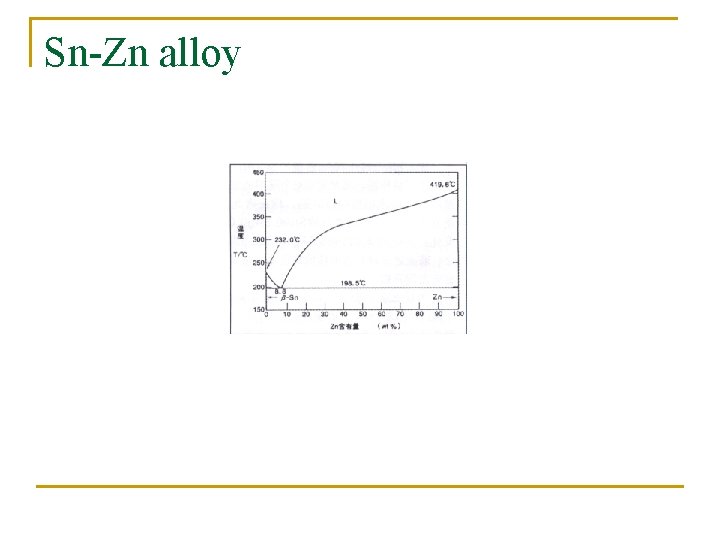
Sn-Zn alloy
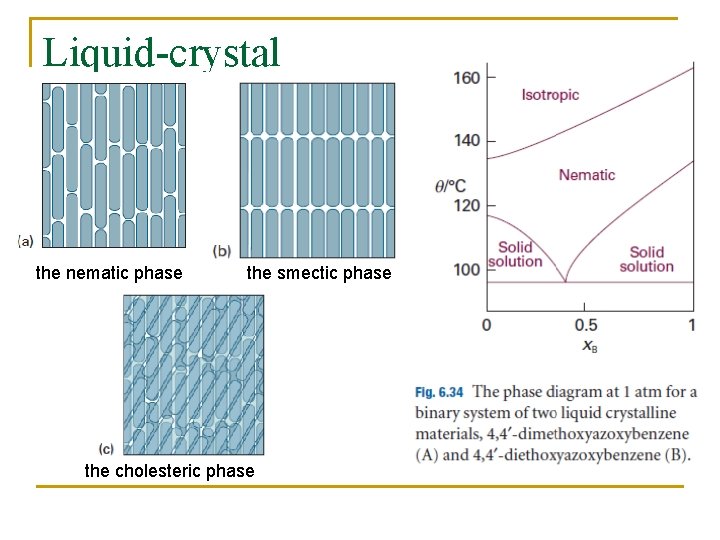
Liquid-crystal the nematic phase the smectic phase the cholesteric phase
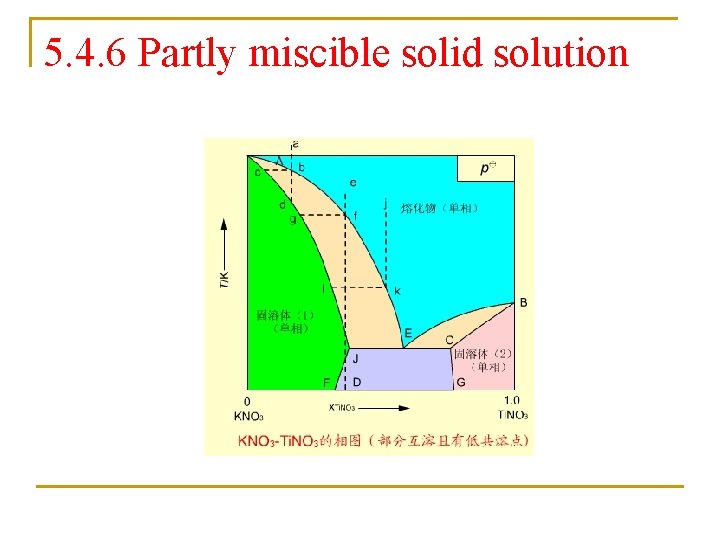
5. 4. 6 Partly miscible solid solution
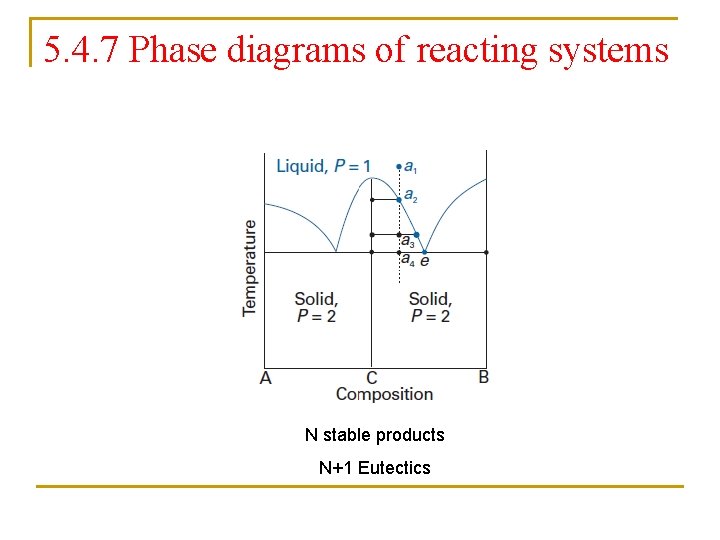
5. 4. 7 Phase diagrams of reacting systems N stable products N+1 Eutectics
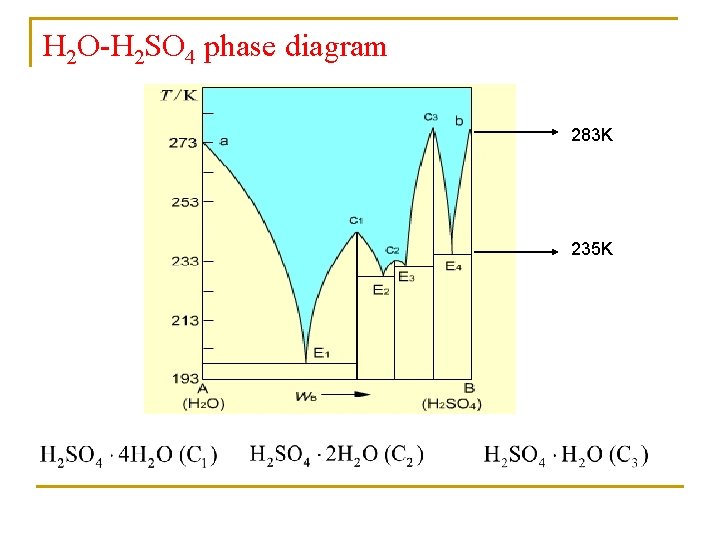
H 2 O-H 2 SO 4 phase diagram 283 K 235 K
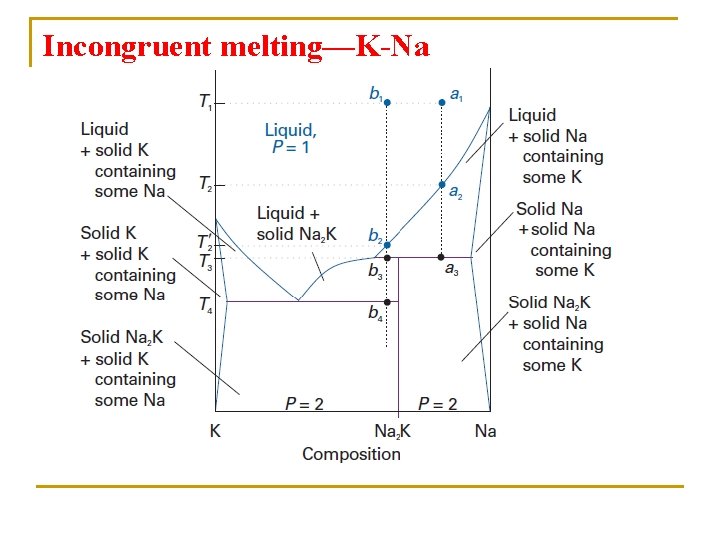
Incongruent melting—K-Na
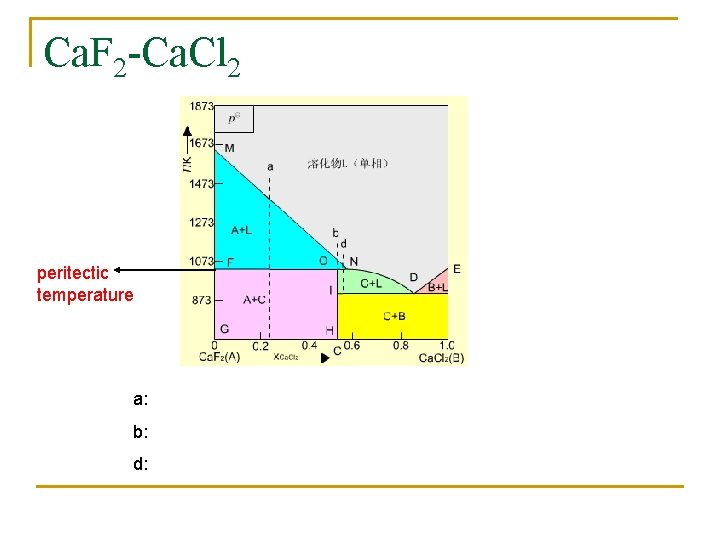
Ca. F 2 -Ca. Cl 2 peritectic temperature a: b: d:
Homework n n n Y: P 176: 28; P 182: 35; P 183: 37 A: P 215: 8. 3(a); P 219: 8. 6 Preview: Y: 5. 10 -5. 12 A:
- Slides: 23