PHASE DIAGRAM BY DR ROZANA AINA MAULAT OSMAN

PHASE DIAGRAM BY: DR. ROZANA AINA MAULAT OSMAN
INTRODUCTION 8 -2 Phase: A phase is a homogeneus, physically distinct and mechanically separable portion of the material with a given chemical composition and structure. For solid – chemically and structurally distinct For liquids – miscibility (mixed) For gases- always 1 phase Phase diagrams: Ø Represents phases present in metal at different conditions (Temperature, pressure and composition). Ø Indicates equilibrium solid solubility of one element in another. Ø Indicates temperature range under which solidification occurs. Ø Indicates temperature at which different phases start to melt.
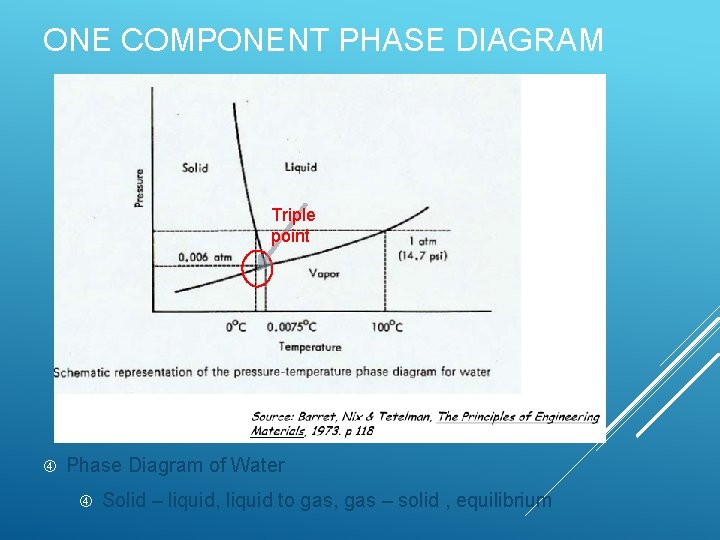
ONE COMPONENT PHASE DIAGRAM Triple point Phase Diagram of Water Solid – liquid, liquid to gas, gas – solid , equilibrium
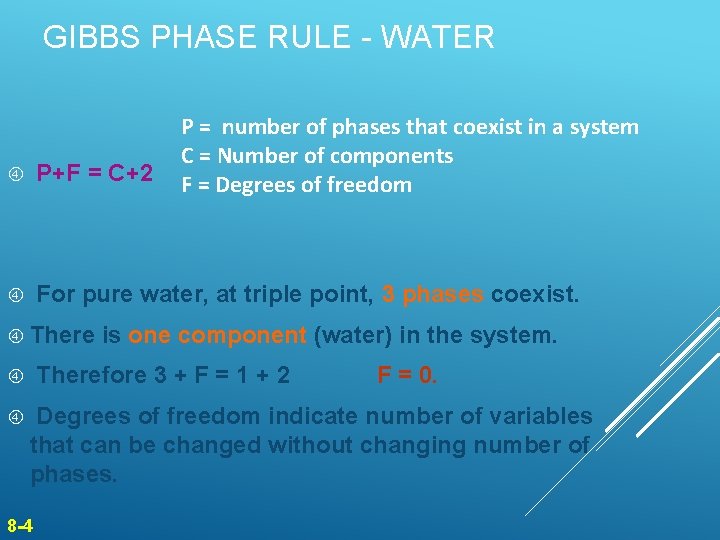
GIBBS PHASE RULE - WATER P = number of phases that coexist in a system C = Number of components F = Degrees of freedom P+F = C+2 For pure water, at triple point, 3 phases coexist. Therefore 3 + F = 1 + 2 is one component (water) in the system. F = 0. Degrees of freedom indicate number of variables that can be changed without changing number of phases. 8 -4
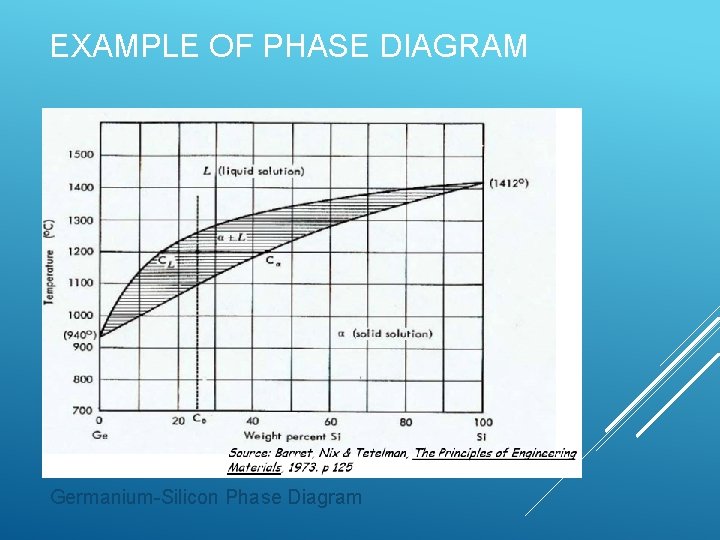
EXAMPLE OF PHASE DIAGRAM Germanium-Silicon Phase Diagram
SOLIDUS AND LIQUIDUS Solidus temperature at which alloy is completely solid Temperature at which liquefaction begins Liquidus Temperature at which alloy is completely liquid Temperature at which solidification begins

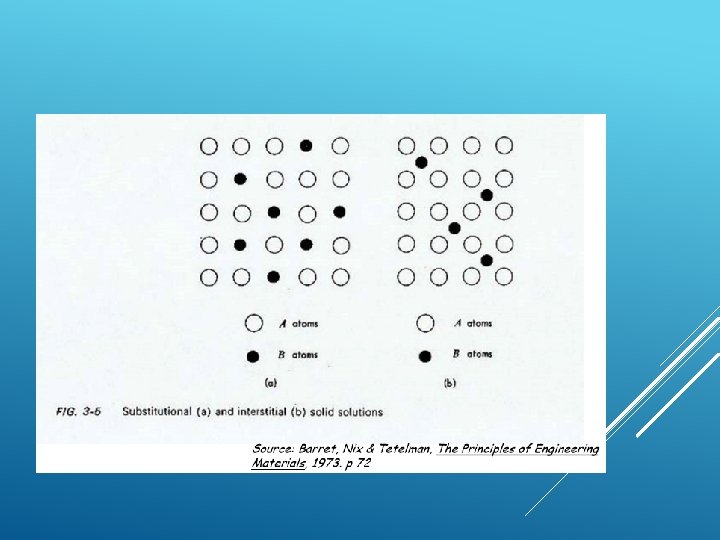
SOLID SOLUTION What is solid solution? When foreign atoms are incorporated into a crystal structure whether in substitutional or interstitial sites, the resulting phase is solid solution of the matrix material (solvent) and the foreign atoms (solute) Substitutional solid solution- Foreign (solute) atoms accupy normal lattice site accupied by matrix ( solvent) atoms, eg: Cu. Ni, Ge-Si Interstitial solid solution- Foreign (solute) atoms occupy interstitial site, eg: Fe-C.
CONT. .
TYPES OF SOLUBILITY Unlimited solid solubility Solute and solvent are mutually soluble at all concentrations, eg: Cu-Ni system Result in a single phase alloy Limited or Partial Solid solubility There a limit to how much of the solute can dissolve in the solvent before saturation is reached, eg: Pb-Sn system Result in multi-phase alloy

Solvent host or major component in solution, solute -minor component. Solubility Limit of a component in a phase is the maximum amount of the component that can be dissolved in it (e. g. alcohol has unlimited solubility in water, sugar has a limited solubility, oil is insoluble). The same concepts apply to solid phases: Cu and Ni are mutuallysoluble in any amount (unlimited solid solubility), while C has a limited solubility in Fe. CONT. .
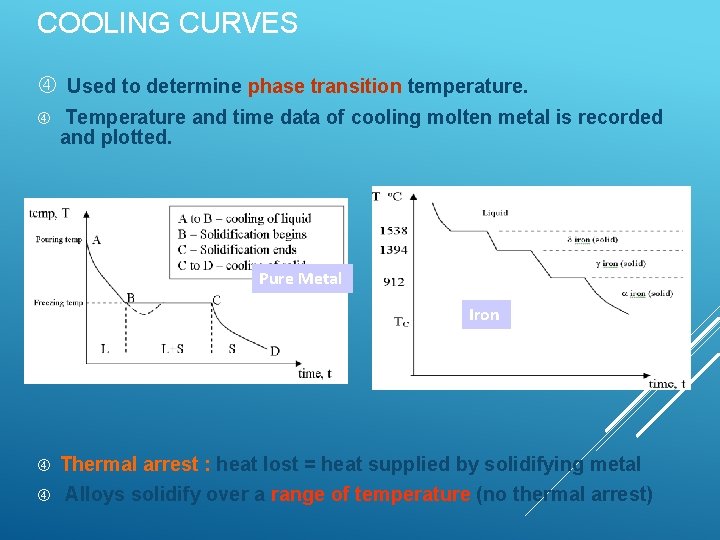
COOLING CURVES Used to determine phase transition temperature. Temperature and time data of cooling molten metal is recorded and plotted. Pure Metal Iron Thermal arrest : heat lost = heat supplied by solidifying metal Alloys solidify over a range of temperature (no thermal arrest)
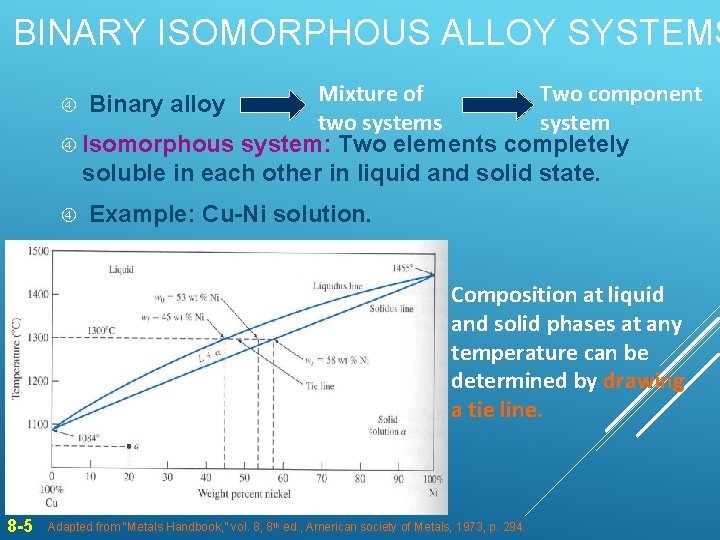
BINARY ISOMORPHOUS ALLOY SYSTEMS Mixture of Two component two systems system Isomorphous system: Two elements completely soluble in each other in liquid and solid state. Binary alloy Example: Cu-Ni solution. Composition at liquid and solid phases at any temperature can be determined by drawing a tie line. 8 -5 Adapted from “Metals Handbook, ” vol. 8, 8 th ed. , American society of Metals, 1973, p. 294.
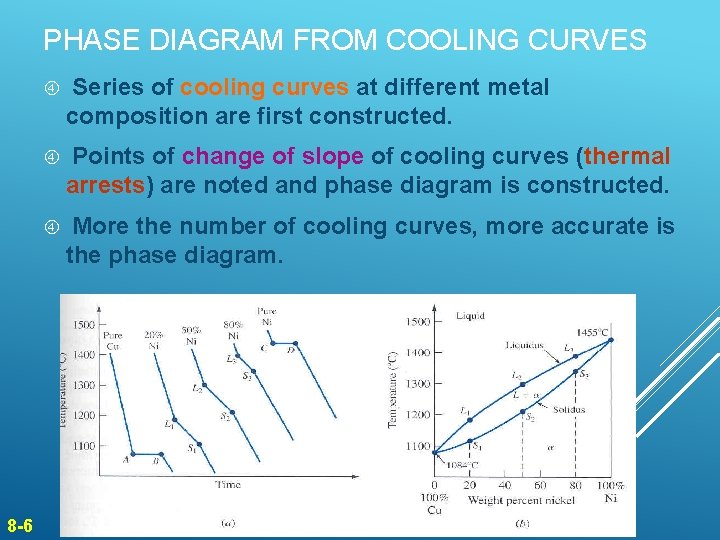
PHASE DIAGRAM FROM COOLING CURVES 8 -6 Series of cooling curves at different metal composition are first constructed. Points of change of slope of cooling curves (thermal arrests) are noted and phase diagram is constructed. More the number of cooling curves, more accurate is the phase diagram.
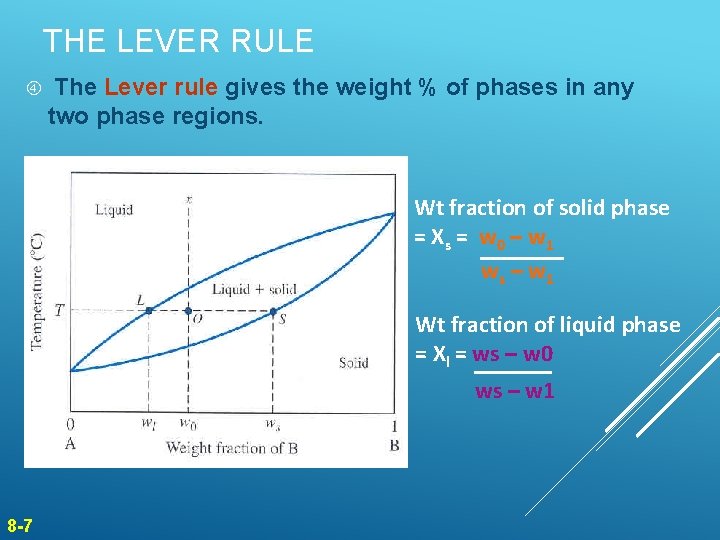
THE LEVER RULE The Lever rule gives the weight % of phases in any two phase regions. Wt fraction of solid phase = X s = w 0 – w 1 ws – w 1 Wt fraction of liquid phase = Xl = ws – w 0 ws – w 1 8 -7
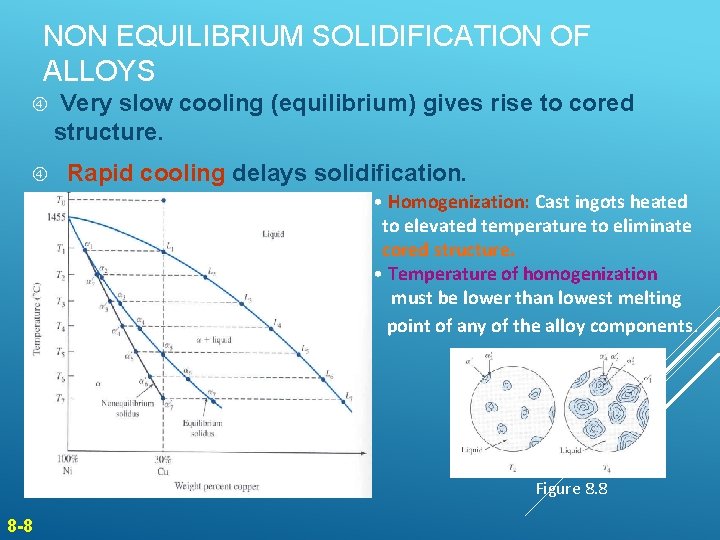
NON EQUILIBRIUM SOLIDIFICATION OF ALLOYS Very slow cooling (equilibrium) gives rise to cored structure. Rapid cooling delays solidification. • Homogenization: Cast ingots heated to elevated temperature to eliminate cored structure. • Temperature of homogenization must be lower than lowest melting point of any of the alloy components. Figure 8. 7 8 -8 Figure 8. 8
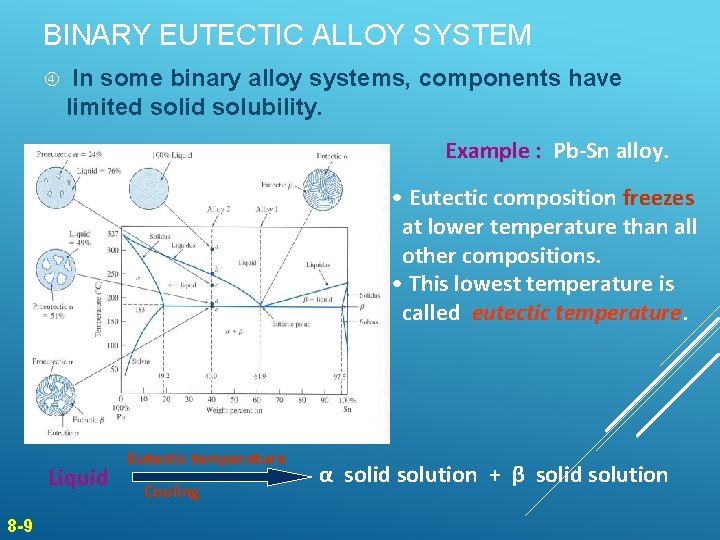
BINARY EUTECTIC ALLOY SYSTEM In some binary alloy systems, components have limited solid solubility. Example : Pb-Sn alloy. • Eutectic composition freezes at lower temperature than all other compositions. • This lowest temperature is called eutectic temperature. Figure 8. 11 Liquid 8 -9 Eutectic temperature Cooling α solid solution + β solid solution
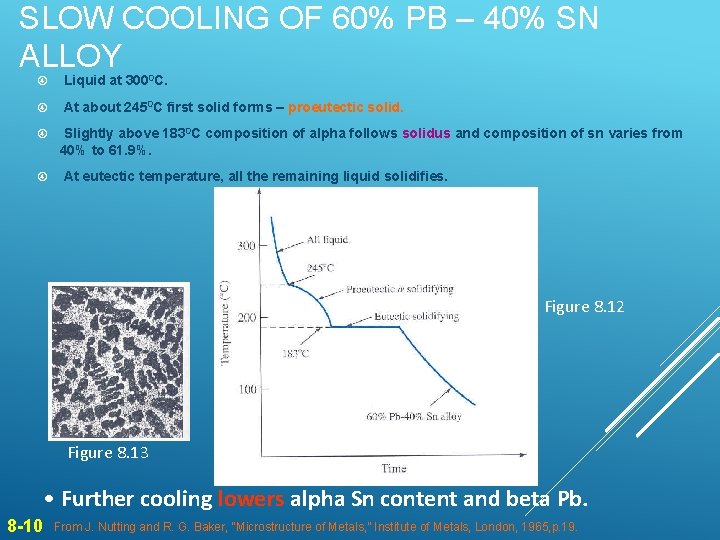
SLOW COOLING OF 60% PB – 40% SN ALLOY Liquid at 3000 C. At about 2450 C first solid forms – proeutectic solid. Slightly above 1830 C composition of alpha follows solidus and composition of sn varies from 40% to 61. 9%. At eutectic temperature, all the remaining liquid solidifies. Figure 8. 12 Figure 8. 13 • Further cooling lowers alpha Sn content and beta Pb. 8 -10 From J. Nutting and R. G. Baker, “Microstructure of Metals, ” Institute of Metals, London, 1965, p. 19.
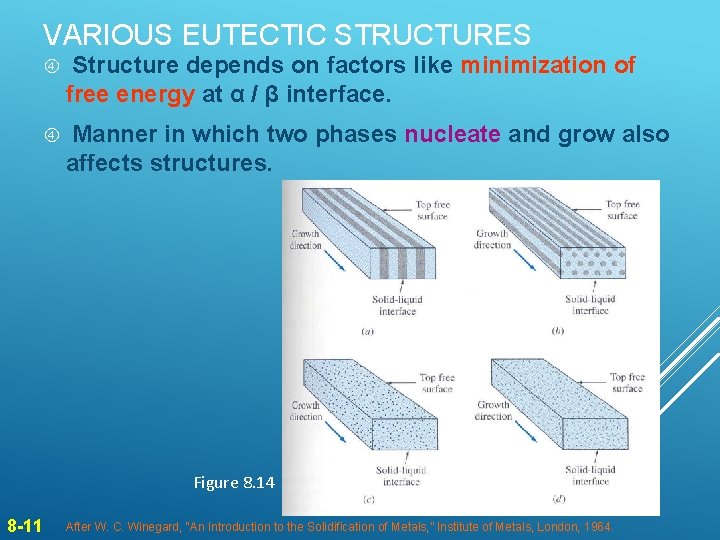
VARIOUS EUTECTIC STRUCTURES Structure depends on factors like minimization of free energy at α / β interface. Manner in which two phases nucleate and grow also affects structures. Figure 8. 14 8 -11 After W. C. Winegard, “An Introduction to the Solidification of Metals, ” Institute of Metals, London, 1964.
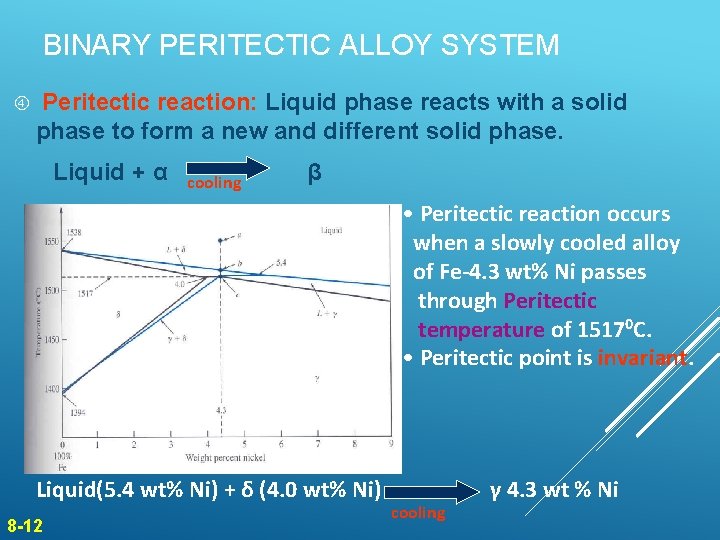
BINARY PERITECTIC ALLOY SYSTEM Peritectic reaction: Liquid phase reacts with a solid phase to form a new and different solid phase. Liquid + α cooling β • Peritectic reaction occurs when a slowly cooled alloy of Fe-4. 3 wt% Ni passes through Peritectic temperature of 15170 C. • Peritectic point is invariant. Figure 8. 16 Liquid(5. 4 wt% Ni) + δ (4. 0 wt% Ni) 8 -12 cooling γ 4. 3 wt % Ni
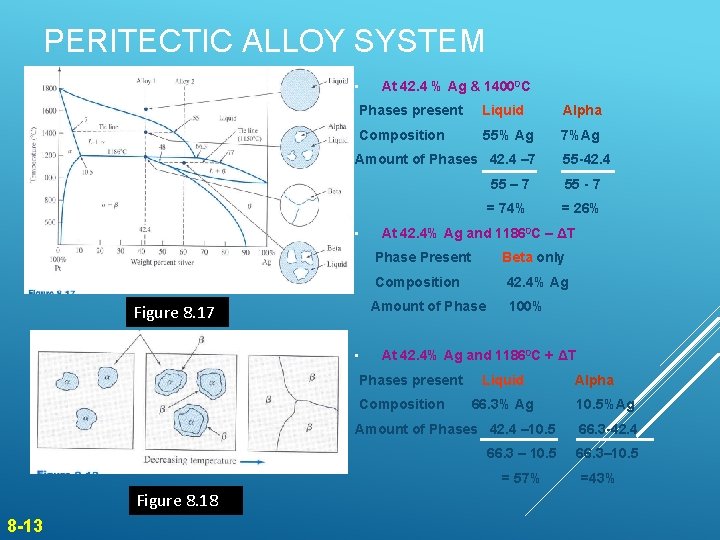
PERITECTIC ALLOY SYSTEM • At 42. 4 % Ag & 14000 C Phases present Liquid Alpha Composition 55% Ag 7%Ag Amount of Phases 42. 4 – 7 • • 55 – 7 55 - 7 = 74% = 26% At 42. 4% Ag and 11860 C – ΔT Phase Present Figure 8. 17 Beta only Composition 42. 4% Ag Amount of Phase 100% At 42. 4% Ag and 11860 C + ΔT Phases present Composition Liquid 66. 3% Ag 8 -13 Alpha 10. 5%Ag Amount of Phases 42. 4 – 10. 5 66. 3 -42. 4 66. 3 – 10. 5 66. 3– 10. 5 = 57% Figure 8. 18 55 -42. 4 =43%
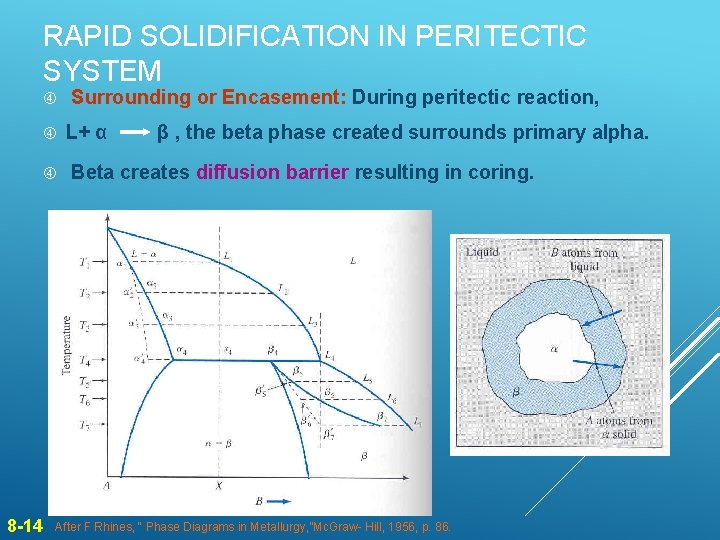
RAPID SOLIDIFICATION IN PERITECTIC SYSTEM 8 -14 Surrounding or Encasement: During peritectic reaction, L+ α β , the beta phase created surrounds primary alpha. Beta creates diffusion barrier resulting in coring. After F Rhines, “ Phase Diagrams in Metallurgy, ”Mc. Graw- Hill, 1956, p. 86.
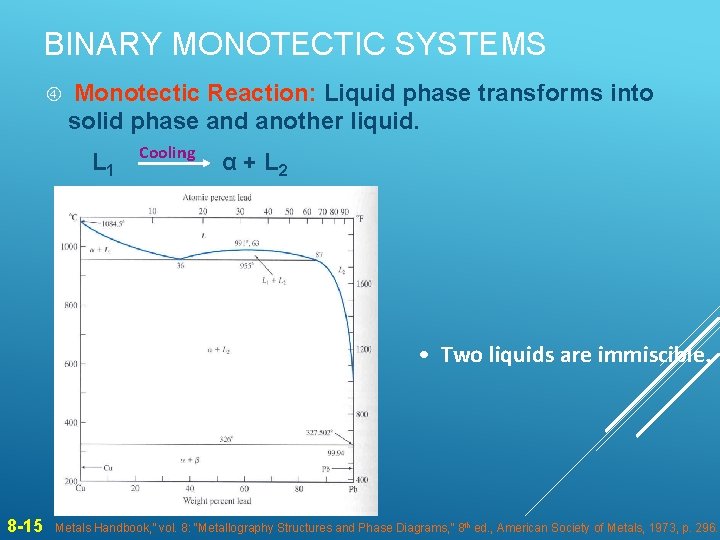
BINARY MONOTECTIC SYSTEMS Monotectic Reaction: Liquid phase transforms into solid phase and another liquid. L 1 Cooling α + L 2 • Two liquids are immiscible. 8 -15 Metals Handbook, ” vol. 8: “Metallography Structures and Phase Diagrams, ” 8 th ed. , American Society of Metals, 1973, p. 296.
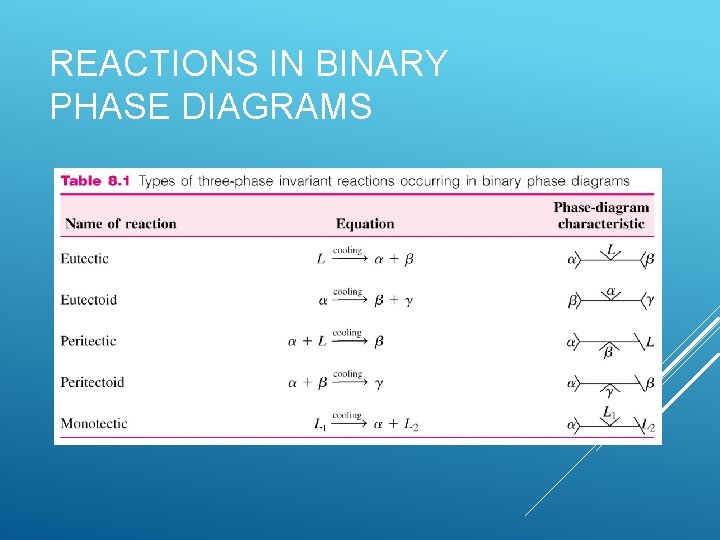
REACTIONS IN BINARY PHASE DIAGRAMS
- Slides: 23