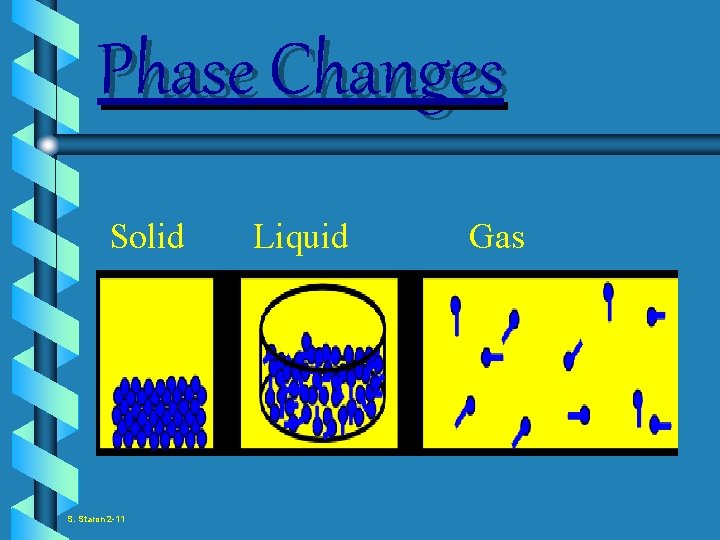
Phase Changes Solid S Staron 2 11 Liquid





















- Slides: 27

Phase Changes Solid S. Staron 2 -11 Liquid Gas

Phase Changes When at least 2 states of matter of same substance are present, each different state is called a PHASE One substance changes from one state of matter to another REVERSIBLE PHYSICAL CHANGE! http: //mutuslab. cs. uwindso r. ca/schurko/animations/w aterphases/status_water. h

Phase Changes A phase change is a reversible physical change that occurs when a substance changes from one state of matter to another. 3

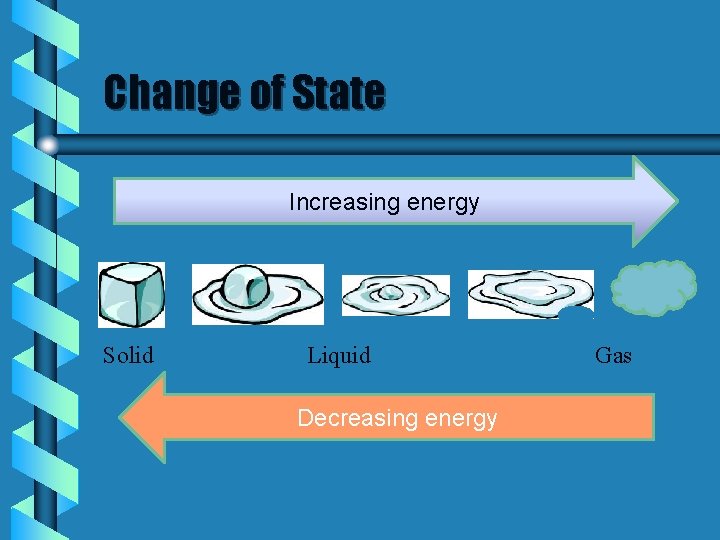
Change of State Increasing energy Solid Liquid Decreasing energy Gas

Phase Change Names Condensation Vaporization Sublimation Deposition Melting Freezing

Phase Changes Require: b Addition of heat energy for melting, vaporization, and sublimation ( s→g) b Removal of heat energy for: freezing, condensation, and deposition (g→s)

Phase Changes b Changing the amount of heat energy usually causes a temperature change. b BUT during a phase change, the temperature stays the same even though the heat energy changes. b This energy goes to changing the phase not into raising the temperature.

REMEMBER THIS! b. The temperature remains constant until the phase change is complete.

Phase Change - Endothermic During an endothermic change – such as melting, the system absorbs energy from its surroundings, and the particles become less orderly.

Phase Change - Vaporization b The phase change in which a substance changes from a liquid into a gas is vaporization b Vaporization is an endothermic process.

Phase Change - Vaporization b. Scientists note differences between two vaporization processes – boiling and evaporation

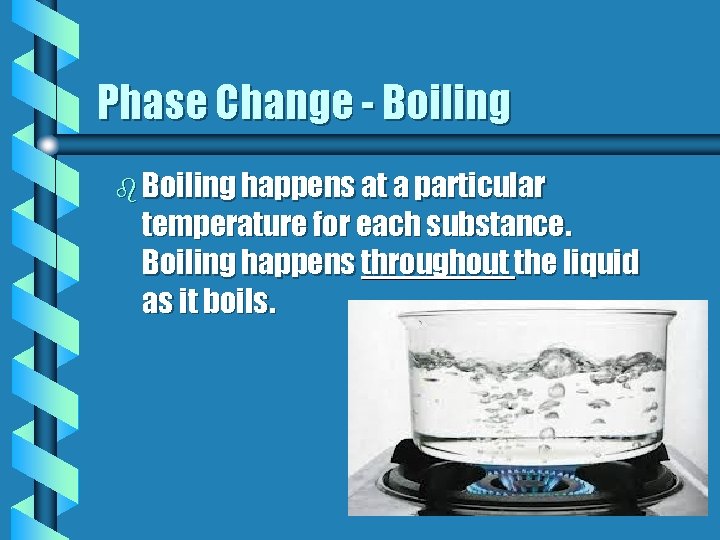
Phase Change - Boiling b Boiling happens at a particular temperature for each substance. Boiling happens throughout the liquid as it boils.

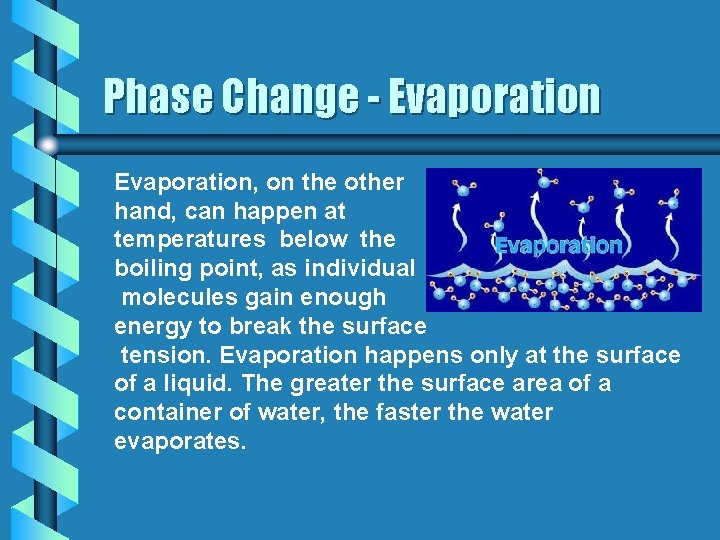
Phase Change - Evaporation, on the other hand, can happen at temperatures below the boiling point, as individual molecules gain enough energy to break the surface tension. Evaporation happens only at the surface of a liquid. The greater the surface area of a container of water, the faster the water evaporates.

Phase Change - Sublimation b Sublimation is the phase change in which a substance changes from a solid to a gas, or vapor, without changing to a liquid first. b Sublimation is an endothermic change.

Phase Change - Exothermic b During an exothermic change such as freezing, the system releases energy to its surroundings. b The arrangement of molecules in water becomes less orderly as ice melts and more orderly as water freezes

Phase Change - Freezing b Freezing is the change from a liquid to a solid, which can happen quite slowly or very quickly.

Phase Change - Condensation b Condensation is the phase change in which a substance changes from a gas, or vapor, to a liquid b Condensation is an exothermic change.

Phase Change - Deposition b Deposition is the phase change in which a gas, or vapor, changes directly into a solid without first changing to a liquid b Deposition is an exothermic change and is the reverse of sublimation

Phase Changes b The temperature of a substance does not change during a phase change. b During a phase change, energy is transferred between a substance and its surroundings.

Melting – Solid to a Liquid Endothermic or Exothermic?

Vaporization – Liquid to a Gas Evaporation – vaporization at the surface of the liquid below boiling point Boiling – vaporization at the boiling point Endothermic or Exothermic?

Solidification – Liquid to a Solid Endothermic or Exothermic?

Condensation – Gas to a Liquid Endothermic or Exothermic?

Sublimation – Solid to a Gas Endothermic or Exothermic?

Deposition – Gas to a Solid Endothermic or Exothermic?

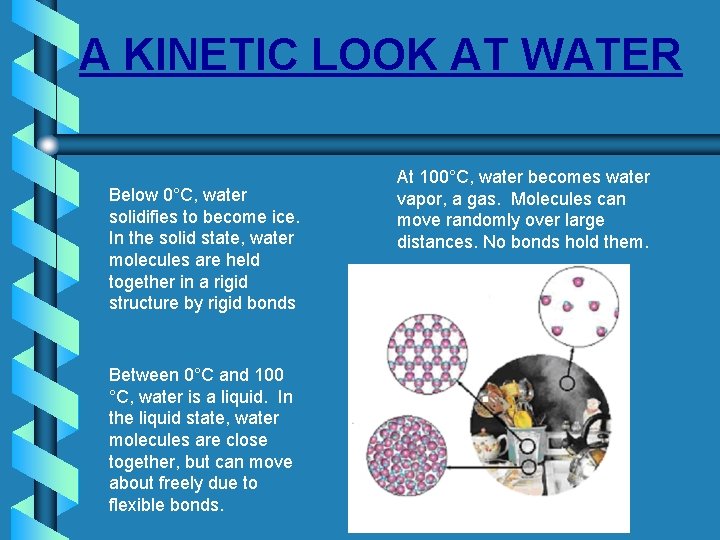
A KINETIC LOOK AT WATER Below 0°C, water solidifies to become ice. In the solid state, water molecules are held together in a rigid structure by rigid bonds Between 0°C and 100 °C, water is a liquid. In the liquid state, water molecules are close together, but can move about freely due to flexible bonds. At 100°C, water becomes water vapor, a gas. Molecules can move randomly over large distances. No bonds hold them.

PHASES MOVIE http: //www. brainpop. com/science/matteran dchemistry/statesofm atter/