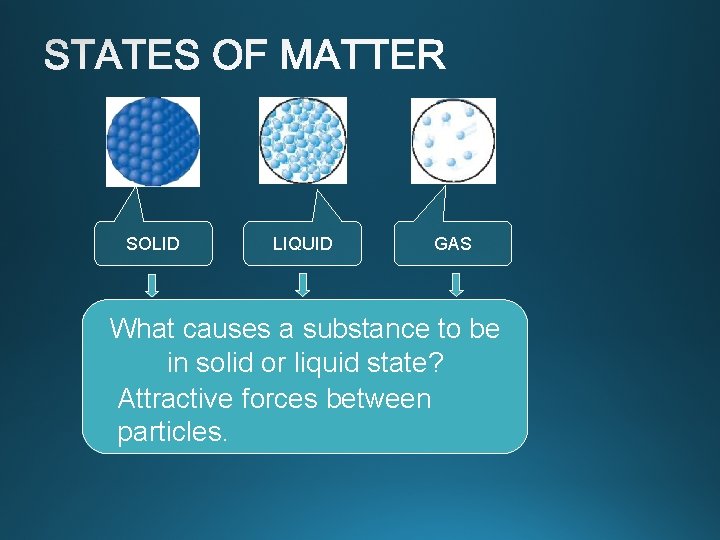
Phase Changes SOLID LIQUID GAS Well separated Close












- Slides: 35

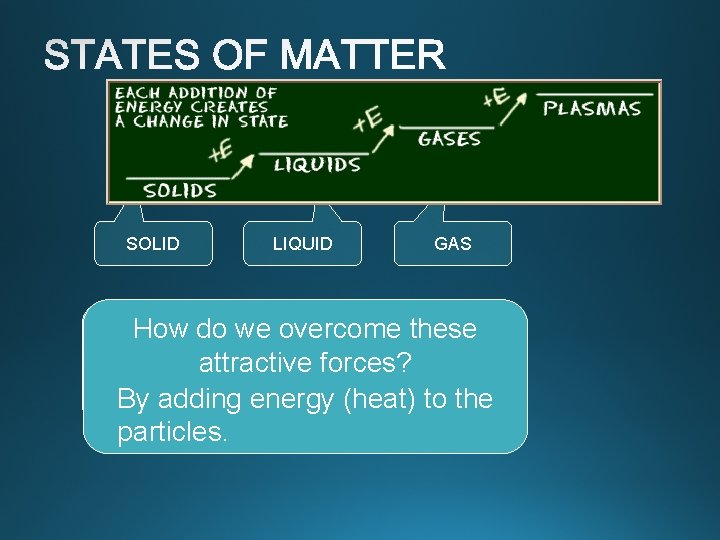
Phase Changes

SOLID LIQUID GAS Well separated Close together What causes a substance be with to no regular with no regular arrangement. in solid Vibrate, or liquid state? Vibrate and move freely at high about, and slide Attractive forces between speeds past each other particles. Tightly packed, in a regular pattern Vibrate, but do not move from place to place

SOLID LIQUID GAS Well separated Close together How do we overcome these with no regular arrangement. attractive forces? Vibrate and move Vibrate, move freely at high about, and slide By adding energy (heat) tospeeds the past each other particles. Tightly packed, in a regular pattern Vibrate, but do not move from place to place





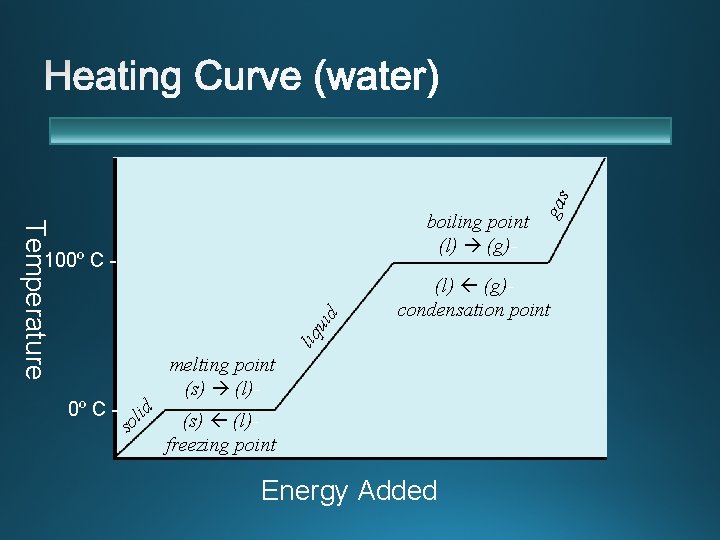
Temperature (l) (g)condensation point liq uid 100º C - s id l o gas boiling point (l) (g)- melting point (s) (l)freezing point Energy Added

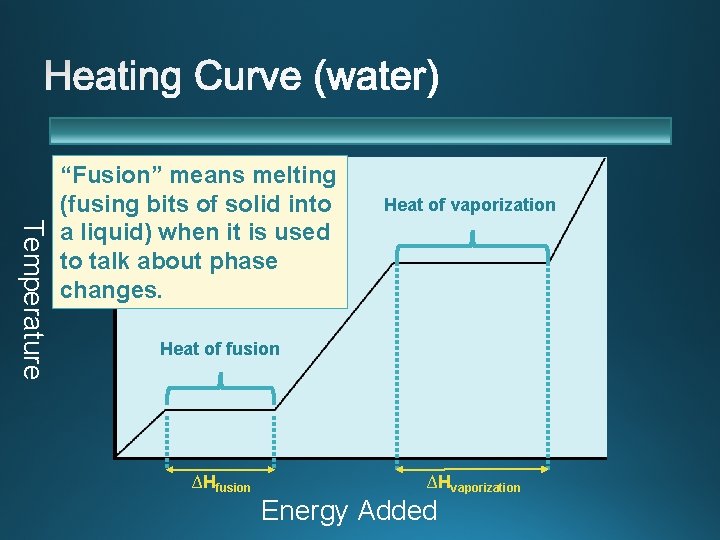
Temperature “Fusion” means melting (fusing bits of solid into a liquid) when it is used to talk about phase changes. Heat of vaporization Heat of fusion ∆Hvaporization Energy Added

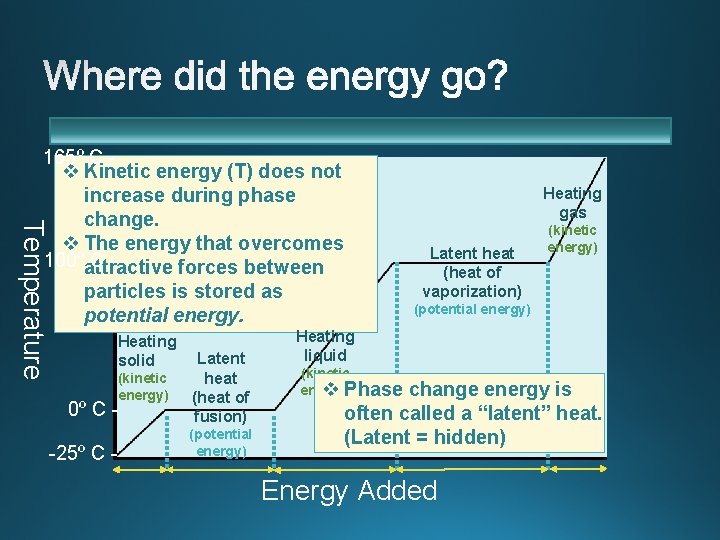
Temperature 165º C v Kinetic energy (T) does not increase during phase change. v The energy that overcomes 100ºattractive Cforces between particles is stored as potential energy. Heating solid 0º C - Latent heat (heat of fusion) -25º C - (potential energy) (kinetic energy) Heating gas Latent heat (heat of vaporization) (kinetic energy) (potential energy) Heating liquid (kinetic energy) v Phase change energy is often called a “latent” heat. (Latent = hidden) Energy Added

v melting point v. Melting Kinetic Energy = temperature

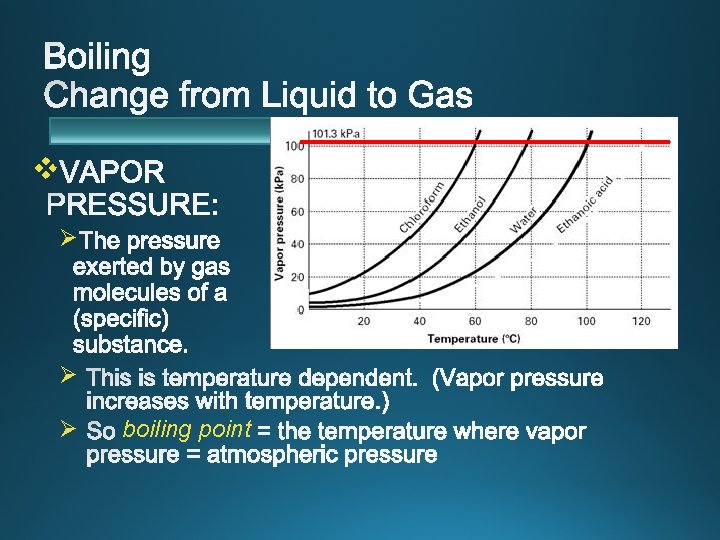
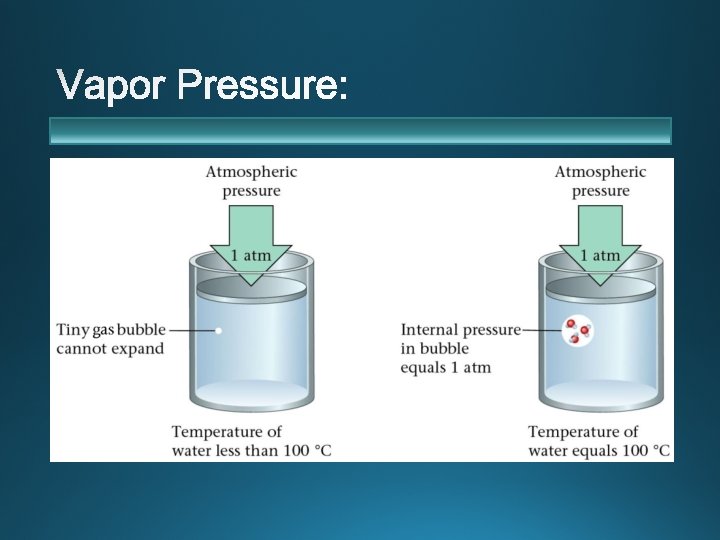
v boiling point v. Boiling pressure vapor

v Ø Ø Ø boiling point


v v

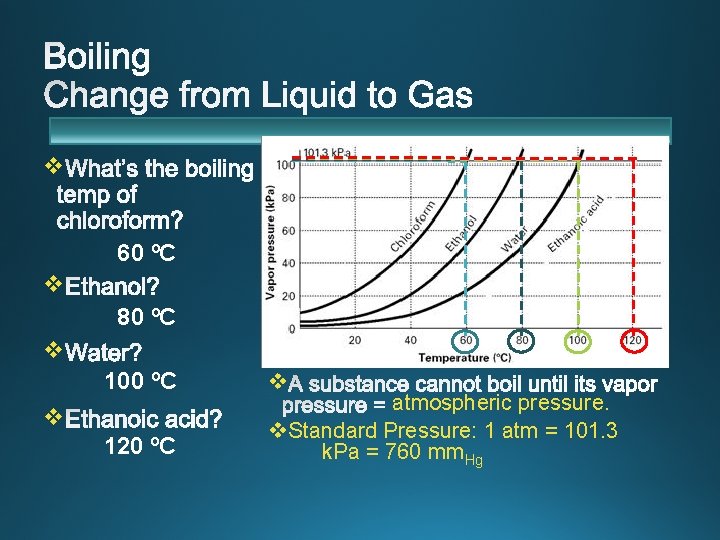
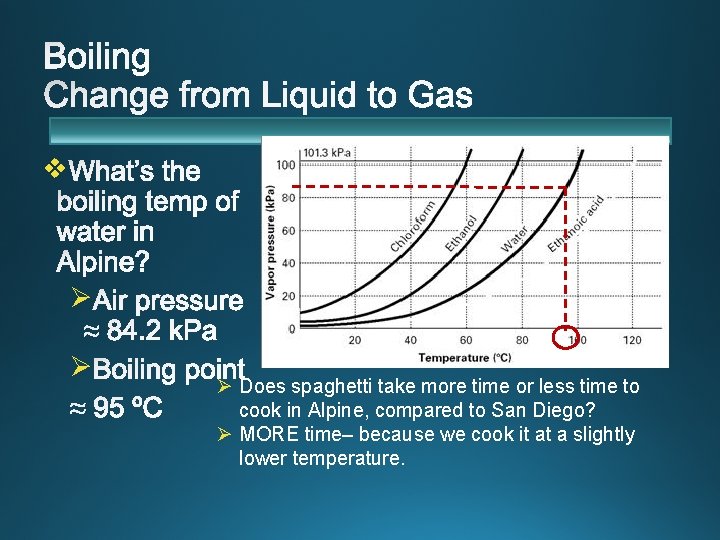
v 60 ºC v 80 ºC v 100 ºC v 120 ºC v atmospheric pressure. v. Standard Pressure: 1 atm = 101. 3 k. Pa = 760 mm. Hg


v Ø Ø Ø Does spaghetti take more time or less time to cook in Alpine, compared to San Diego? Ø MORE time– because we cook it at a slightly lower temperature.

v v Ø Pressure Ø Temperature

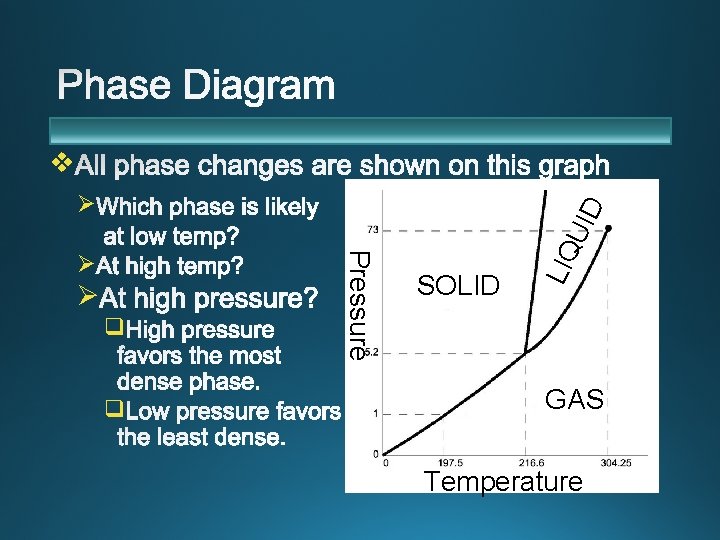
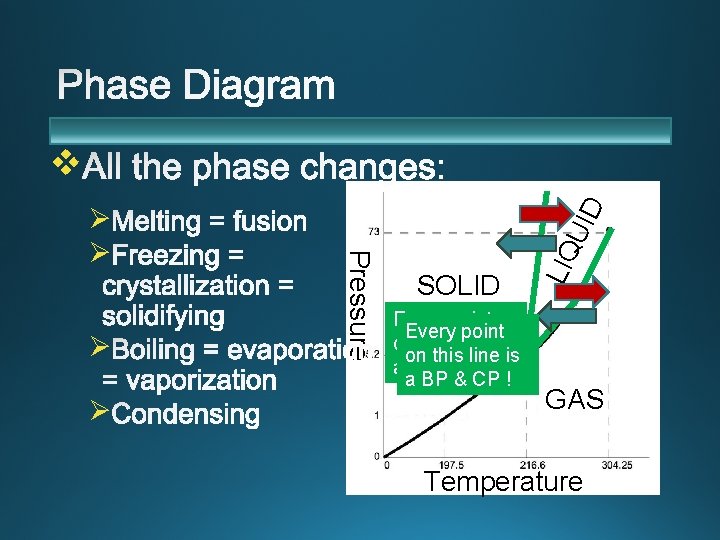
v Ø q q Pressure Ø SOLID LIQ UID Ø GAS Temperature

Ø Ø SOLID Every point on this line is a MP & FP ! a BP & CP ! LIQ Pressure Ø Ø UID v GAS Temperature

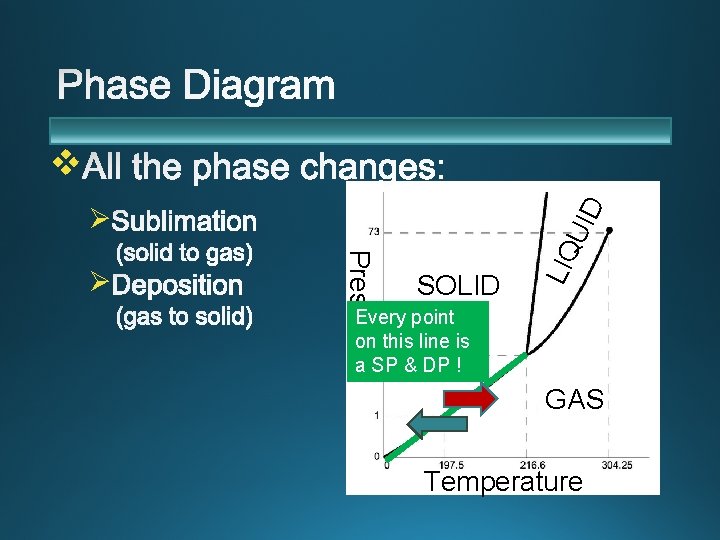
v SOLID LIQ Pressure Ø UID Ø Every point on this line is a SP & DP ! GAS Temperature

Requires the input of energy Examples: dry ice, iodine and snow Sublimation of snow occurs more readily under at high altitudes with less air pressure, with dry winds. Sublimation occurs (on a small scale) in your freezer– when things get freezer burn.

(The opposite of sublimation) Ø Requires the release of energy. Ø Deposition commonly occurs when water vapor freezes to form snow or frost. Ø (On your windshield, for example. ) Ø This also occurs in your freezer with the freezer burn! Deposition of iodine

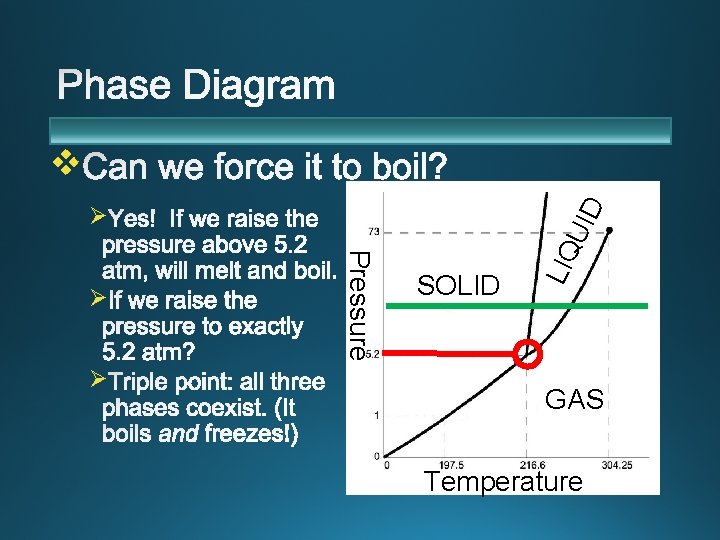
Ø SOLID LIQ Pressure Ø Standard Pressure Ø UID v GAS Temperature

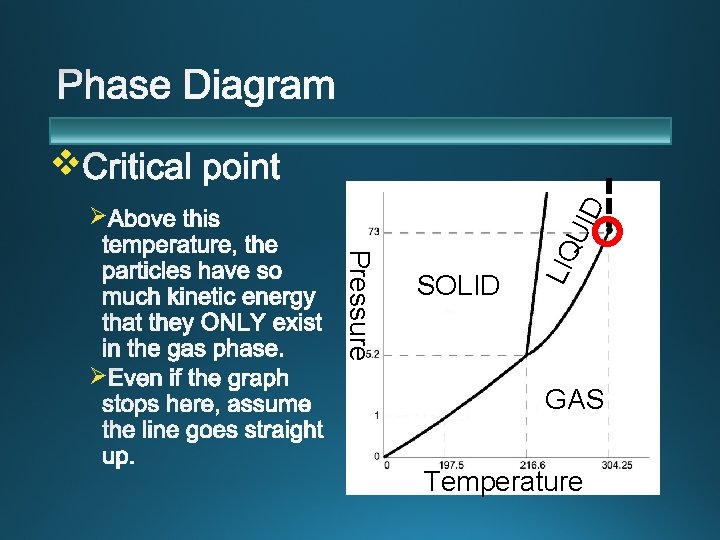
v Ø SOLID LIQ Pressure Ø UID Ø GAS Temperature

v SOLID LIQ Pressure Ø UID Ø GAS Temperature

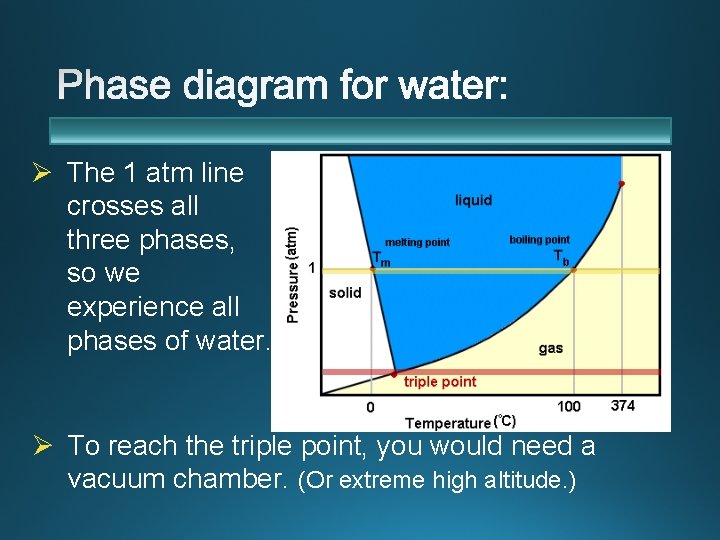
Ø The 1 atm line crosses all three phases, so we experience all phases of water. Ø To reach the triple point, you would need a vacuum chamber. (Or extreme high altitude. )

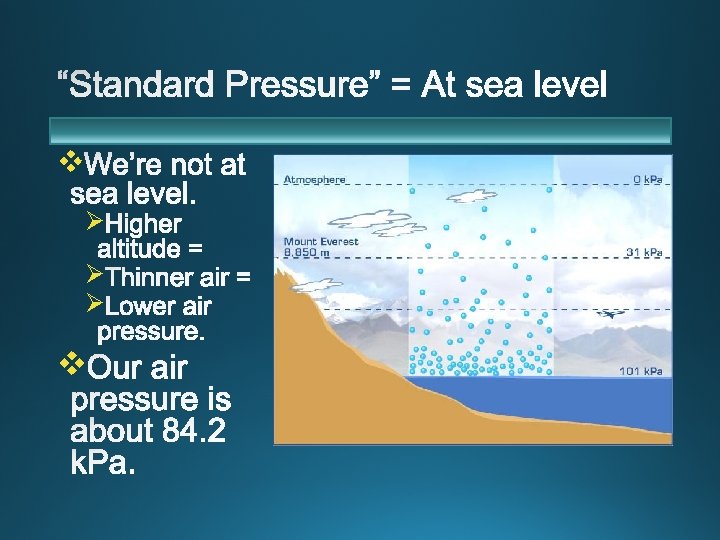
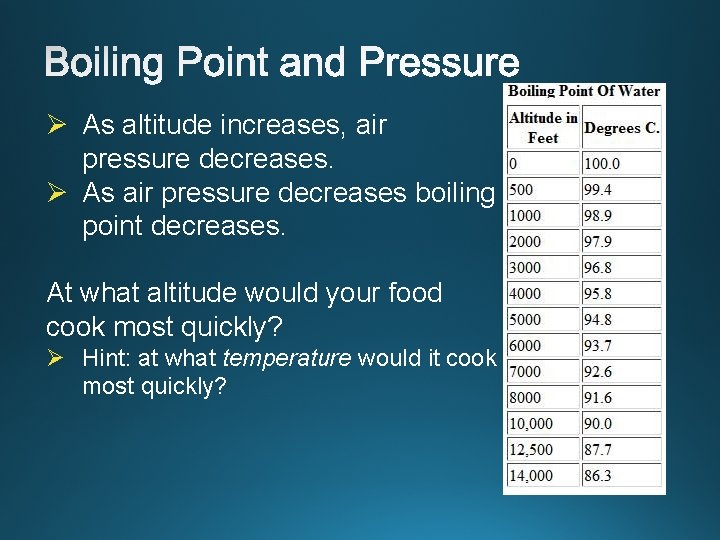
Ø As altitude increases, air pressure decreases. Ø As air pressure decreases boiling point decreases. At what altitude would your food cook most quickly? Ø Hint: at what temperature would it cook most quickly?

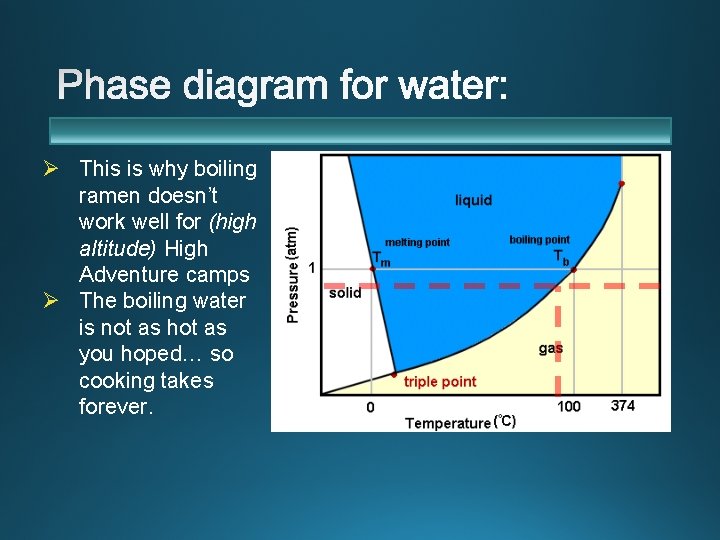
Ø This is why boiling ramen doesn’t work well for (high altitude) High Adventure camps Ø The boiling water is not as hot as you hoped… so cooking takes forever.

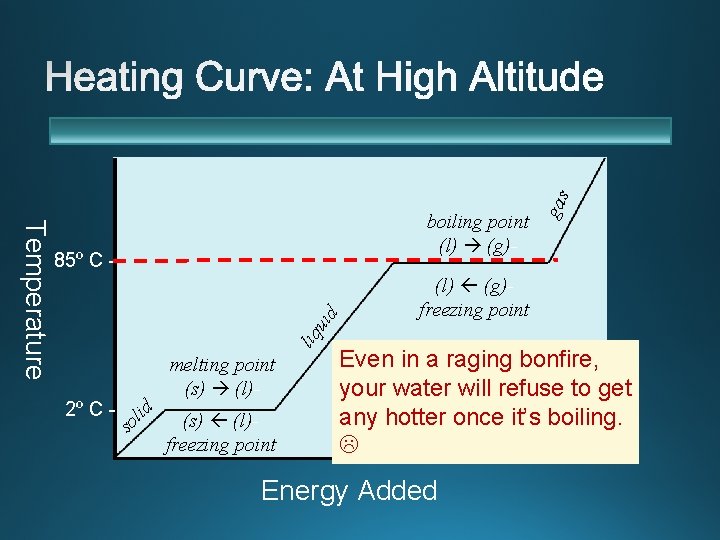
uid 85º C - liq Temperature 2º C - s id l o melting point (s) (l)freezing point gas boiling point (l) (g)freezing point Even in a raging bonfire, your water will refuse to get any hotter once it’s boiling. Energy Added

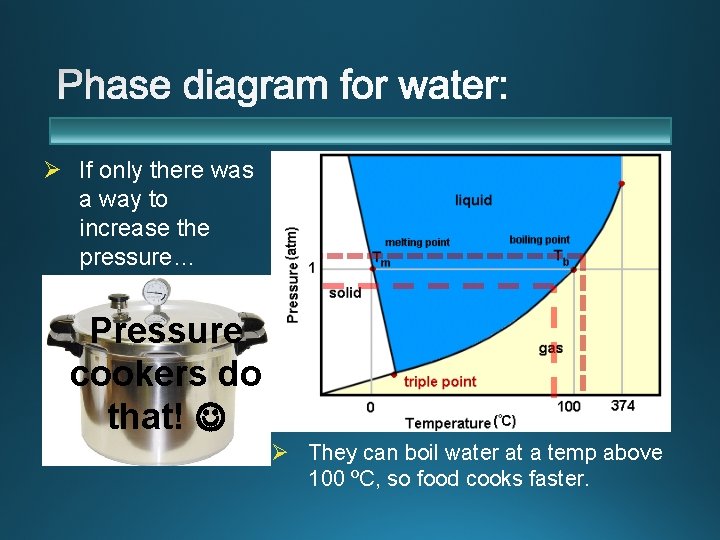
Ø If only there was a way to increase the pressure… Pressure cookers do that! Ø They can boil water at a temp above 100 ºC, so food cooks faster.

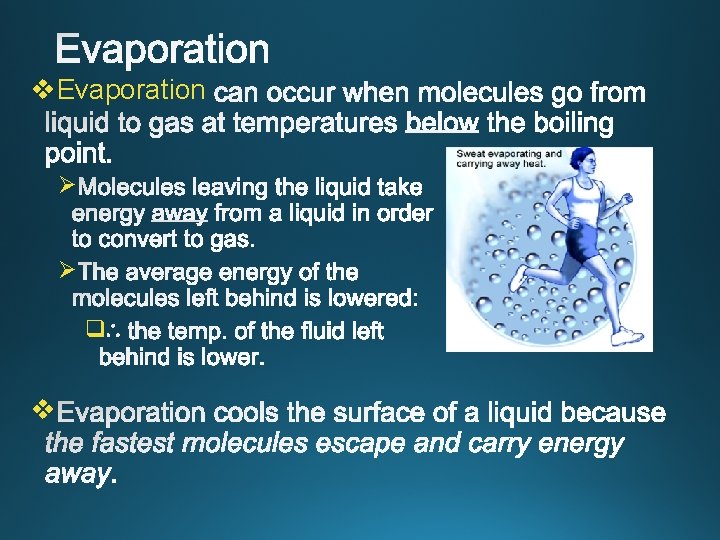
v. Evaporation Ø Ø q v

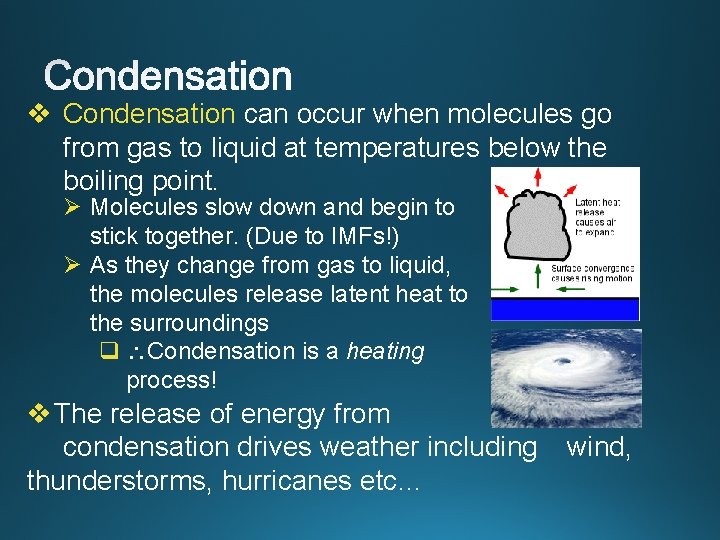
v Condensation can occur when molecules go from gas to liquid at temperatures below the boiling point. Ø Molecules slow down and begin to stick together. (Due to IMFs!) Ø As they change from gas to liquid, the molecules release latent heat to the surroundings q Condensation is a heating process! v The release of energy from condensation drives weather including thunderstorms, hurricanes etc… wind,
