Phase 12 Primary Analysis of ZUMA6 Axicabtagene Ciloleucel

Phase 1/2 Primary Analysis of ZUMA-6: Axicabtagene Ciloleucel in Combination With Atezolizumab for the Treatment of Patients With Refractory Diffuse Large B Cell Lymphoma Caron A. Jacobson, MD, MMSc 1; Jason R. Westin, MD, MS 2; David B. Miklos, MD, Ph. D 3; Alex F. Herrera, MD 4; Jennifer Lee, BS 5; Judy Seng, BS 5; John M. Rossi, MS 5; Jennifer Sun, MS 5; Jinghui Dong, Ph. D 5; Zachary J. Roberts, MD, Ph. D 5; Remus Vezan, MD, Ph. D 5; Mauro P. Avanzi, MD, Ph. D 5; and Frederick L. Locke, MD 6 1 Dana-Farber Cancer Institute, Boston, MA, USA; 2 University of Texas MD Anderson Cancer Center, Houston, TX, USA; 3 Stanford University School of Medicine, Stanford, CA, USA; 4 City of Hope National Medical Center, Duarte, CA, USA; 5 Kite, a Gilead Company, Santa Monica, CA, USA; and 6 Moffitt Cancer Center, Tampa, FL, USA DE‐CTH‐ 2020‐ 05‐ 0010 (May 2020)

Disclosures Honoraria from Kite, a Gilead Company, Novartis, Pfizer, Celgene, Humanigen, Precision Biosciences, and Nkarta; consultancy or advisory role for Kite, a Gilead Company, Novartis, Celgene, Pfizer, Humanigen, Precision Biosciences, and Nkarta; speakers' bureau participation for Axis and Clinical Care Options; research funding from Pfizer; and travel support from Kite, a Gilead Company, Celgene, Novartis, Pfizer, Humanigen, and Precision Biosciences.

Disclosures Disclaimer EMA approval for axi‐cel is for the treatment of adult patients with relapsed or refractory diffuse large B‐cell lymphoma (DLBCL) and primary mediastinal large B‐ cell lymphoma (PMBCL), after two or more lines of systemic therapy. 1 These slides may contain information that is not within EMA approved product labeling and has not otherwise been approved by the EMA. 1 Yescarta® (axicabtagene ciloleucel) Sm. PC (January 2020)
Background • Axicabtagene ciloleucel is a US‐ and EU‐approved autologous anti‐CD 19 CAR T cell therapy for the treatment of adult relapsed/refractory LBCL after ≥ 2 prior lines of therapy 1 - In the ZUMA‐ 1 pivotal study, the ORR was 83%, with a CR rate of 58% 2 • Checkpoint proteins (eg, PD‐ 1, PD‐L 1) have been shown to be upregulated within the tumor and after CAR T cell infusion 3‐ 6 - Checkpoint blockade may augment activity of CAR T cells • Atezolizumab is an engineered, humanized monoclonal antibody that binds to PD‐L 1 to activate the antitumor immune response 7 • ZUMA‐ 6 is a Phase 1/2, open‐label, multicenter study evaluating axi‐cel in combination with atezo in patients with refractory DLBCL 1. YESCARTA® (axicabtagene ciloleucel) [EMA Sm. PC]. https: //www. ema. europa. eu/en/medicines/human/EPAR/yescarta#product‐information‐section. 2. Locke FL, et al. Lancet Oncol. 2019; 20: 31‐ 42. 3. Perez A, et al. Presented at ASH 2015. #2042. 4. Galon J, et al. Presented at ASCO 2017. #3025. 5. Neelapu SS, et al. Presented at ASH 2017. #578. 6. Arihara Y, et al. Presented at SITC 2019. #P 210. 7. TECENTRIQ® (atezolizumab) [EMA Sm. PC]. https: //www. ema. europa. eu/en/medicines/human/EPAR/tecentriq#product‐information‐section Atezo, atezolizumab; axi‐cel, axicabtagene ciloleucel; CAR, chimeric antigen receptor; CR, complete response; DLBCL, diffuse large B cell lymphoma; LBCL, large B cell lymphoma; ORR, objective response rate; sc. Fv, single‐chain variable fragment. Jacobson et al AACR 2020 Abstract #8872 4
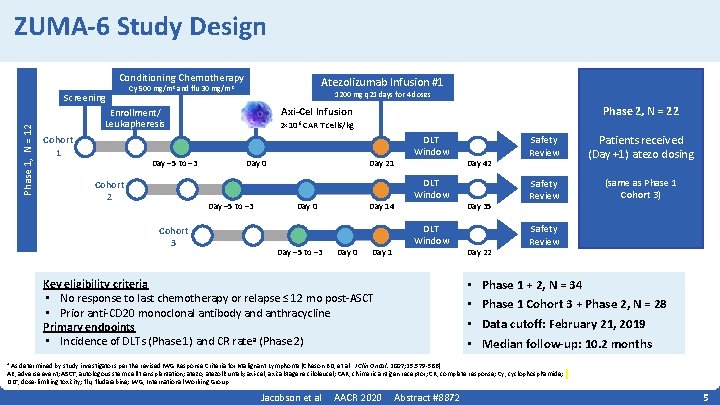
ZUMA-6 Study Design Conditioning Chemotherapy Phase 1, N = 12 Screening Atezolizumab Infusion #1 Cy 500 mg/m 2 and flu 30 mg/m 2 1200 mg q 21 days for 4 doses Cohort 1 Day − 5 to − 3 Cohort 2 2× 106 CAR T cells/kg Day 0 Day − 5 to − 3 Cohort 3 Phase 2, N = 22 Axi-Cel Infusion Enrollment/ Leukapheresis Day 21 Day 0 Day − 5 to − 3 Day 14 Day 0 Day 1 DLT Window Key eligibility criteria • No response to last chemotherapy or relapse ≤ 12 mo post‐ASCT • Prior anti‐CD 20 monoclonal antibody and anthracycline Primary endpoints • Incidence of DLTs (Phase 1) and CR ratea (Phase 2) Day 42 Day 35 Day 22 • • Safety Review Patients received (Day +1) atezo dosing Safety Review (same as Phase 1 Cohort 3) Safety Review Phase 1 + 2, N = 34 Phase 1 Cohort 3 + Phase 2, N = 28 Data cutoff: February 21, 2019 Median follow-up: 10. 2 months As determined by study investigators per the revised IWG Response Criteria for Malignant Lymphoma (Cheson BD, et al. J Clin Oncol. 2007; 25: 579‐ 586). AE, adverse event; ASCT, autologous stem cell transplantation; atezo, atezolizumab; axi‐cel, axicabtagene ciloleucel; CAR, chimeric antigen receptor; CR, complete response; Cy, cyclophosphamide; DLT, dose‐limiting toxicity; flu, fludarabine; IWG, International Working Group. a Jacobson et al AACR 2020 Abstract #8872 5
Baseline Disease Characteristics Phase 1 Cohort 3 + Phase 2 (N = 28) 58 (42 – 71) 16 (57) n (%) Median age (range), years Male, n (%) ECOG performance status, n (%) 0 1 Disease stagea, n (%) II III or IV IPI score, n (%) 0– 2 3– 4 ≥ 2 | ≥ 3 prior therapies, n (%) Primary refractory disease, n (%) 17 (61) 11 (39) 6 (21) 22 (79) 15 (54) 13 (46) 24 (86) | 14 (50) 4 (14) • 34 patients received axi‐cel and ≥ 1 atezo dose, and 24/34 patients (71%) received all 4 atezo doses No patients had disease stage I. Atezo, atezolizumab; axi‐cel, axicabtagene ciloleucel; ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index. a Jacobson et al AACR 2020 Abstract #8872 6
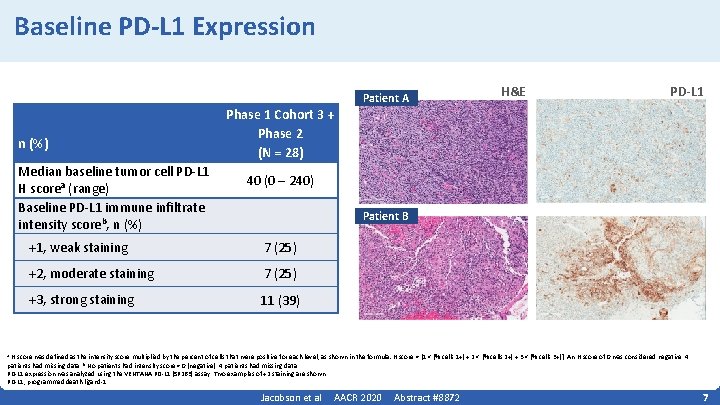
Baseline PD-L 1 Expression n (%) Median baseline tumor cell PD‐L 1 H scorea (range) Baseline PD‐L 1 immune infiltrate intensity scoreb, n (%) Phase 1 Cohort 3 + Phase 2 (N = 28) Patient A H&E PD-L 1 40 (0 – 240) Patient B +1, weak staining 7 (25) +2, moderate staining 7 (25) +3, strong staining 11 (39) H score was defined as the intensity score multiplied by the percent of cells that were positive for each level, as shown in the formula: H score = [1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3+)]. An H score of 0 was considered negative. 4 patients had missing data. b No patients had intensity score = 0 (negative). 4 patients had missing data. PD‐L 1 expression was analyzed using the VENTANA PD‐L 1 (SP 263) assay. Two examples of +2 staining are shown. PD‐L 1, programmed death ligand‐ 1. a Jacobson et al AACR 2020 Abstract #8872 7
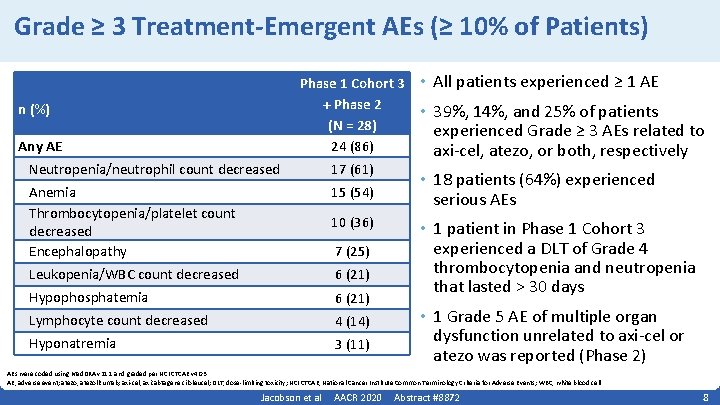
Grade ≥ 3 Treatment-Emergent AEs (≥ 10% of Patients) Phase 1 Cohort 3 + Phase 2 (N = 28) 24 (86) n (%) Any AE Neutropenia/neutrophil count decreased 17 (61) Anemia Thrombocytopenia/platelet count decreased Encephalopathy 15 (54) Leukopenia/WBC count decreased 6 (21) Hypophosphatemia 6 (21) Lymphocyte count decreased 4 (14) Hyponatremia 3 (11) 10 (36) 7 (25) • All patients experienced ≥ 1 AE • 39%, 14%, and 25% of patients experienced Grade ≥ 3 AEs related to axi‐cel, atezo, or both, respectively • 18 patients (64%) experienced serious AEs • 1 patient in Phase 1 Cohort 3 experienced a DLT of Grade 4 thrombocytopenia and neutropenia that lasted > 30 days • 1 Grade 5 AE of multiple organ dysfunction unrelated to axi‐cel or atezo was reported (Phase 2) AEs were coded using Med. DRA v 21. 1 and graded per NCI CTCAE v 4. 03. AE, adverse event; atezo, atezolizumab; axi‐cel, axicabtagene ciloleucel; DLT, dose‐limiting toxicity; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; WBC, white blood cell. Jacobson et al AACR 2020 Abstract #8872 8
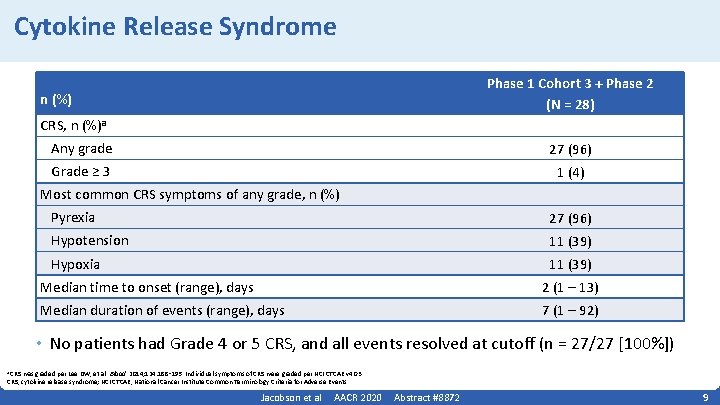
Cytokine Release Syndrome Phase 1 Cohort 3 + Phase 2 (N = 28) n (%) CRS, n (%)a Any grade 27 (96) Grade ≥ 3 1 (4) Most common CRS symptoms of any grade, n (%) Pyrexia 27 (96) Hypotension 11 (39) Hypoxia 11 (39) Median time to onset (range), days 2 (1 – 13) Median duration of events (range), days 7 (1 – 92) • No patients had Grade 4 or 5 CRS, and all events resolved at cutoff (n = 27/27 [100%]) a CRS was graded per Lee DW, et al. Blood. 2014; 124: 188‑ 195. Individual symptoms of CRS were graded per NCI CTCAE v 4. 03. CRS, cytokine release syndrome; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events. Jacobson et al AACR 2020 Abstract #8872 9
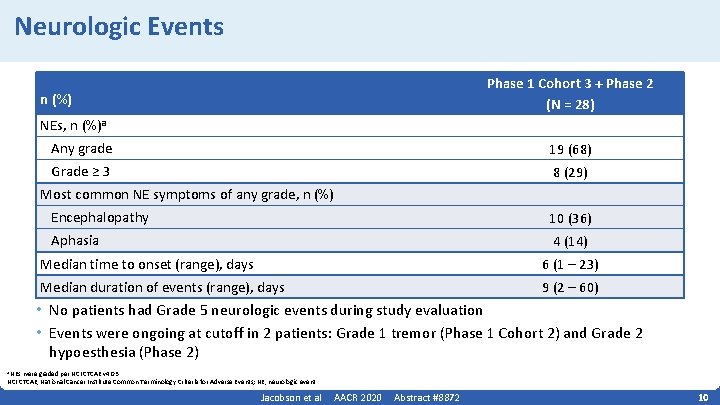
Neurologic Events Phase 1 Cohort 3 + Phase 2 (N = 28) n (%) NEs, n (%)a Any grade 19 (68) Grade ≥ 3 8 (29) Most common NE symptoms of any grade, n (%) Encephalopathy 10 (36) Aphasia 4 (14) Median time to onset (range), days 6 (1 – 23) Median duration of events (range), days 9 (2 – 60) • No patients had Grade 5 neurologic events during study evaluation • Events were ongoing at cutoff in 2 patients: Grade 1 tremor (Phase 1 Cohort 2) and Grade 2 hypoesthesia (Phase 2) a NEs were graded per NCI CTCAE v 4. 03. NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; NE, neurologic event. Jacobson et al AACR 2020 Abstract #8872 10
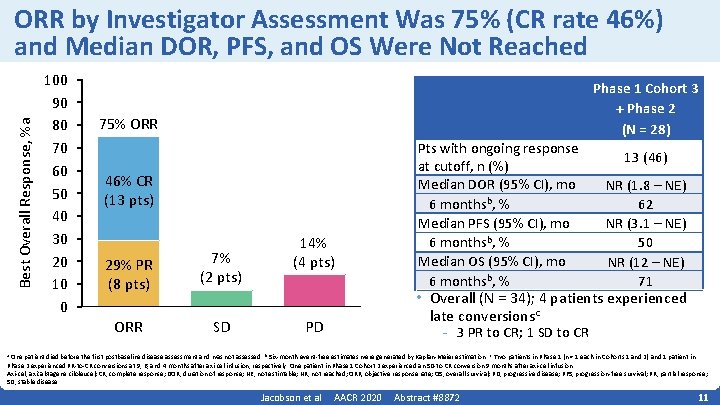
Best Overall Response, %a ORR by Investigator Assessment Was 75% (CR rate 46%) and Median DOR, PFS, and OS Were Not Reached 100 90 80 70 60 50 40 30 20 10 0 Phase 1 Cohort 3 + Phase 2 (N = 28) 75% ORR 46% CR (13 pts) 29% PR (8 pts) 7% (2 pts) ORR SD 14% (4 pts) Pts with ongoing response at cutoff, n (%) Median DOR (95% CI), mo 6 monthsb, % Median PFS (95% CI), mo 6 monthsb, % Median OS (95% CI), mo 6 monthsb, % 13 (46) NR (1. 8 – NE) 62 NR (3. 1 – NE) 50 NR (12 – NE) 71 • Overall (N = 34); 4 patients experienced late conversionsc PD - 3 PR to CR; 1 SD to CR One patient died before the first postbaseline disease assessment and was not assessed. b Six‐month event‐free estimates were generated by Kaplan‐Meier estimation. c Two patients in Phase 1 (n = 1 each in Cohorts 1 and 2) and 1 patient in Phase 2 experienced PR‐to‐CR conversions at 9, 6, and 4 months after axi‐cel infusion, respectively. One patient in Phase 1 Cohort 2 experienced an SD‐to‐CR conversion 9 months after axi‐cel infusion. Axi‐cel, axicabtagene ciloleucel; CR, complete response; DOR, duration of response; NE, not estimable; NR, not reached; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease. a Jacobson et al AACR 2020 Abstract #8872 11
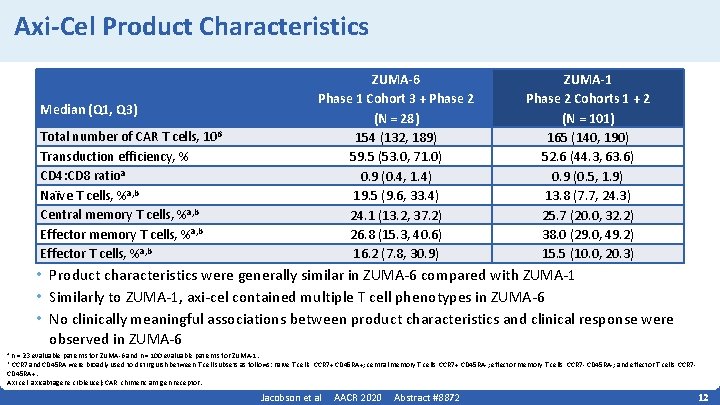
Axi-Cel Product Characteristics Median (Q 1, Q 3) Total number of CAR T cells, 106 Transduction efficiency, % CD 4: CD 8 ratioa Naïve T cells, %a, b Central memory T cells, %a, b Effector T cells, %a, b ZUMA-6 Phase 1 Cohort 3 + Phase 2 (N = 28) 154 (132, 189) 59. 5 (53. 0, 71. 0) 0. 9 (0. 4, 1. 4) 19. 5 (9. 6, 33. 4) 24. 1 (13. 2, 37. 2) 26. 8 (15. 3, 40. 6) 16. 2 (7. 8, 30. 9) ZUMA-1 Phase 2 Cohorts 1 + 2 (N = 101) 165 (140, 190) 52. 6 (44. 3, 63. 6) 0. 9 (0. 5, 1. 9) 13. 8 (7. 7, 24. 3) 25. 7 (20. 0, 32. 2) 38. 0 (29. 0, 49. 2) 15. 5 (10. 0, 20. 3) • Product characteristics were generally similar in ZUMA‐ 6 compared with ZUMA‐ 1 • Similarly to ZUMA‐ 1, axi‐cel contained multiple T cell phenotypes in ZUMA‐ 6 • No clinically meaningful associations between product characteristics and clinical response were observed in ZUMA‐ 6 n = 23 evaluable patients for ZUMA‐ 6 and n = 100 evaluable patients for ZUMA‐ 1. CCR 7 and CD 45 RA were broadly used to distinguish between T cell subsets as follows: naïve T cells, CCR 7+ CD 45 RA+; central memory T cells, CCR 7+ CD 45 RA‐; effector memory T cells, CCR 7‐ CD 45 RA‐; and effector T cells, CCR 7‐ CD 45 RA+. Axi‐cel, axicabtagene ciloleucel; CAR, chimeric antigen receptor. a b Jacobson et al AACR 2020 Abstract #8872 12
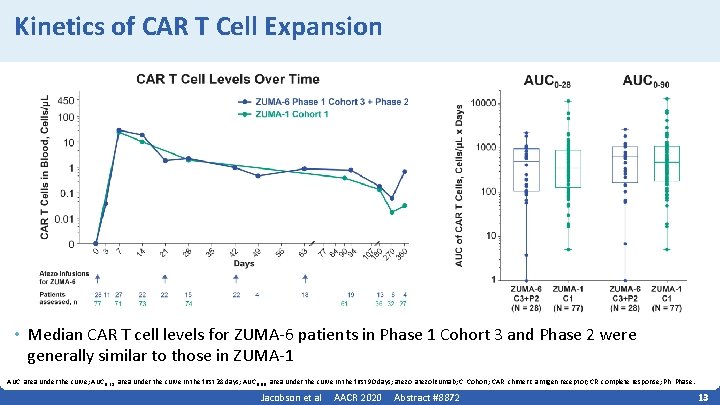
Kinetics of CAR T Cell Expansion • Median CAR T cell levels for ZUMA‐ 6 patients in Phase 1 Cohort 3 and Phase 2 were generally similar to those in ZUMA‐ 1 AUC, area under the curve; AUC 0‐ 28, area under the curve in the first 28 days; AUC 0‐ 90, area under the curve in the first 90 days; atezo, atezolizumab; C, Cohort; CAR, chimeric antigen receptor; CR, complete response; Ph, Phase. Jacobson et al AACR 2020 Abstract #8872 13
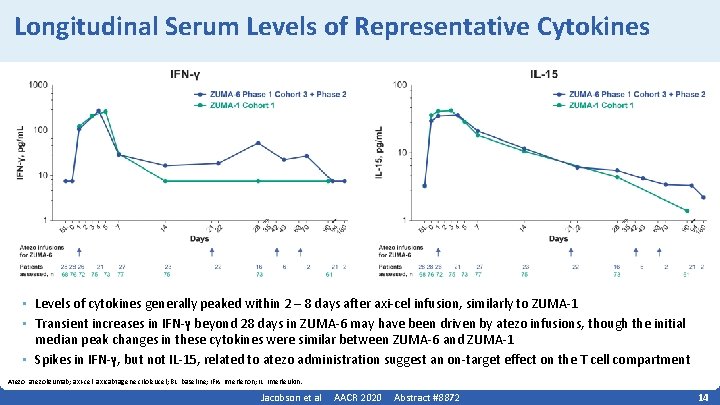
Longitudinal Serum Levels of Representative Cytokines • Levels of cytokines generally peaked within 2 – 8 days after axi‐cel infusion, similarly to ZUMA‐ 1 • Transient increases in IFN‐γ beyond 28 days in ZUMA‐ 6 may have been driven by atezo infusions, though the initial median peak changes in these cytokines were similar between ZUMA‐ 6 and ZUMA‐ 1 • Spikes in IFN‐γ, but not IL‐ 15, related to atezo administration suggest an on‐target effect on the T cell compartment Atezo, atezolizumab; axi‐cel, axicabtagene ciloleucel; BL, baseline; IFN, interferon; IL, interleukin. Jacobson et al AACR 2020 Abstract #8872 14
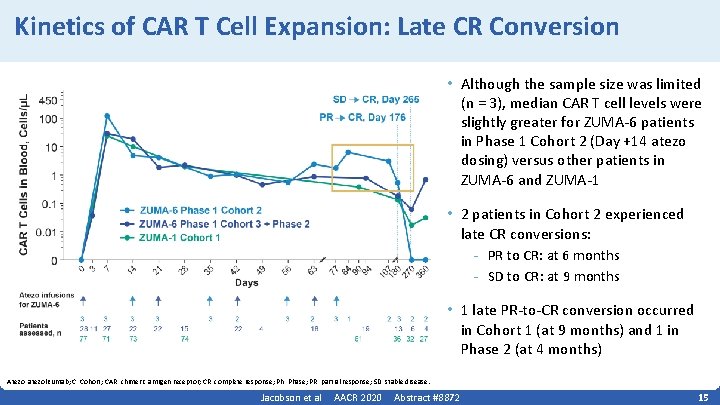
Kinetics of CAR T Cell Expansion: Late CR Conversion • Although the sample size was limited (n = 3), median CAR T cell levels were slightly greater for ZUMA‐ 6 patients in Phase 1 Cohort 2 (Day +14 atezo dosing) versus other patients in ZUMA‐ 6 and ZUMA‐ 1 • 2 patients in Cohort 2 experienced late CR conversions: - PR to CR: at 6 months - SD to CR: at 9 months • 1 late PR‐to‐CR conversion occurred in Cohort 1 (at 9 months) and 1 in Phase 2 (at 4 months) Atezo, atezolizumab; C, Cohort; CAR, chimeric antigen receptor; CR, complete response; Ph, Phase; PR, partial response; SD, stable disease. Jacobson et al AACR 2020 Abstract #8872 15
Conclusions • PD‐L 1 blockade with atezo after axi‐cel has a manageable safety profile consistent with that observed in ZUMA‐ 1, with no significant evidence of increased incidence of AEs - No deaths due to CRS or neurologic events; most symptoms occurred early in treatment and were generally reversible • Efficacy outcomes of axi‐cel in combination with atezo appeared to be comparable with those observed in patients treated with axi‐cel alone • CAR T cell PK and PD parameters were qualitatively similar to those observed in ZUMA‐ 1 • Though the sample size is small (n = 3), CAR T cell persistence beyond 28 days appeared greater in ZUMA‐ 6 Phase 1 Cohort 2 (Day +14 atezo dosing) versus ZUMA‐ 1 - Further studies of axi‐cel in combination with atezo may be needed to establish an interaction between timing of checkpoint blockade and CAR T cell treatment AE, adverse event; atezo, atezolizumab; axi‐cel, axicabtagene ciloleucel; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; PD, pharmacodynamic; PK, pharmacokinetic. Jacobson et al AACR 2020 Abstract #8872 16

Acknowledgments • The patients, families, friends, and caregivers • The study staff • Medical writing support was provided by Ashley Skorusa, Ph. D, of Nexus Global Group Science, funded by Kite, a Gilead Company Jacobson et al AACR 2020 Abstract #8872 17
- Slides: 17