Pharmacology Pharmacology The study of drugs and their









































- Slides: 45

Pharmacology

Pharmacology • The study of drugs and their actions

Reasons For Medication Use • • • Diagnose Treatment Curative Lesson pain Prevent a disease or condition

Where Do Drugs Come From? • Plants • Animals • Minerals • Laboratory Chemical combinations • Biotechnology Genetics

Pharmacokinetics Definition: • The process a drug goes through upon entering the body • It involves the processes of absorption, distribution, biotransformation (metabolism - liver), and excretion

Pharmacokinetics Absorption: For a drug to work , it must be absorbed. It is absorbed at the site where it is given. Once a drug reaches the capillaries, it is taken into the bloodstream. The rate of absorption varies depending on the type of drug, amount of the drug, route, and the patient.

Pharmacokinetics Continued Distribution: How the drug gets to its target once it is in the bloodstream. Involves circulation of the blood. Targets are cells in tissue or organs.

Pharmacokinetics Metabolism: How a drug is broken down for excretion. Products of breakdown are called metabolites. Metabolites are rendered small, inactive or less active before excretion occurs. Metabolism usually occurs in the liver, however can occur in the kidneys, lungs, blood plasma, or intestinal mucosa.

Pharmacokinetics Excretion: A drug continues to have an action until it is excreted by the body. Kidneys generally are the excretory route. Drugs can be excreted by saliva, sweat, exhalation, breast milk, or feces (poop).

Pharmacodynamics Definition: Is the study of how a drug acts on targeted cells.

Pharmacodynamics Types of Drug Actions: • Inhibitive or destructive • Protection • Supplementation • Replacement • Physiologic function (increase or decrease)

Pharmacodynamics Drug Effect or Action Theories: • Agonists- Drug binds to a special receptor on a target cell and produce the desired effect. • Antagonists- Drug binds with a special receptor on a target cell and prevent or inhibit a response. • Non-specific- Neither agonists or antagonists. These drugs gather on a cell’s membrane or go through it and interfere with actual cell function

Pharmacodynamics Timing of Drug Effects: • Onset- time from when a drug is given (administered) to when effects first occur (or are noticed) • Peak Effect- when a drug is at its maximum (peak) effect • Duration- time from when drug effects begin to when they stop

Routes of Drug Administration • • • Buccal(*topical) - cheek IM Inhalation Intra-articular Intradermal/dermal Intrathecal – anywhere CSF flows *Topical =‘s any epithelial lined surface. Tongue, rectally, cheek, vagina etc IV Intracardiac po/PO SC/subq - Subcuticular(within epidermis) vs subcutaneous(below skin) sublingual (topical) – under the tongue *topical

Drug Preparations • • • Forms of: solid semi-solid liquid gas/vapors

Abbreviations for PO or Topical Administration • • Topical cream gtts (ear) - drops ung (ointment) • • PO cap gtts (oral/infants) soln/sol - A solution is a mixture in which other substances are dissolved. • Susp - A suspension is a mixture of liquids with particles of a solid which may not dissolve in the liquid. • Tab - tablet

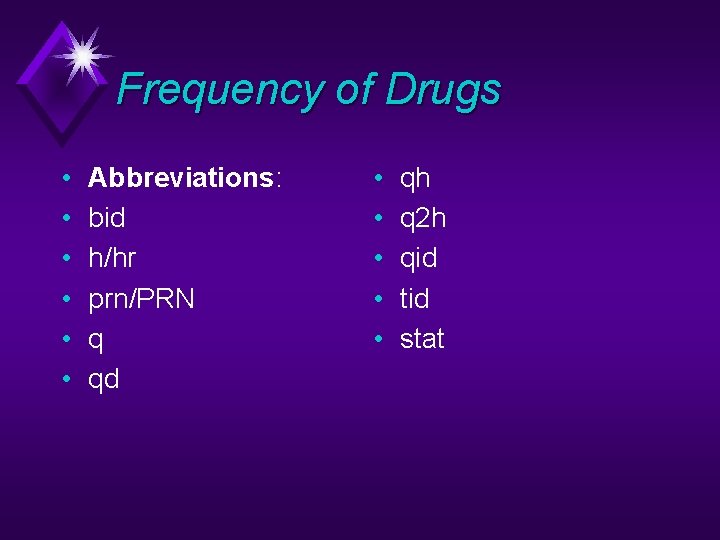
Frequency of Drugs • • • Abbreviations: bid h/hr prn/PRN q qd • • • qh q 2 h qid tid stat

Administration Abbreviations • • AD (right ear) AS AU OD (right eye) OS OU os aa (of each) • • ad (towards) RX c (line over it) dc/DC KVO/TKO npo/NPO per

Nomenclature (Naming) of Drugs • Chemical name: chemical make-up of a drug • Generic name: nonproprietary name given to a drug by the developer of a drug (acetaminophen, ibuprofen, diphenhydramine) • Trade or Brand name: Proprietary or patented name of the manufacturer of the drug (Tylenol, Motrin & Advil, Benedryl). Manufacturing processes may differ slightly.

Legal Implications (Federal) • Pure Food and Drug Act -Standards for US marketing • Federal Food, Drug, and Cosmetic Act -Regulations -FDA approval • Controlled Substances Act -Established DEA -Schedule of Controlled Substances: C-I - Marijuana C-IV – Phenobarbital (treat seizures) C-II - Cocaine C-V – antidiarrheal (low abuse rate) C-III – anabolic steroids The lower the # the higher the addiction/abuse potential

Legal Implications (State) • Regulate practice acts for ordering, prescribing, and administration of medications • As a surgical technologist, we act as an extension of the physician handling drugs under their supervision

Legal Implications (Local) • May have specific guidelines for medication administration in the institution you are employed in

Metric System • Meter = length • Liter = capacity • Gram = weight

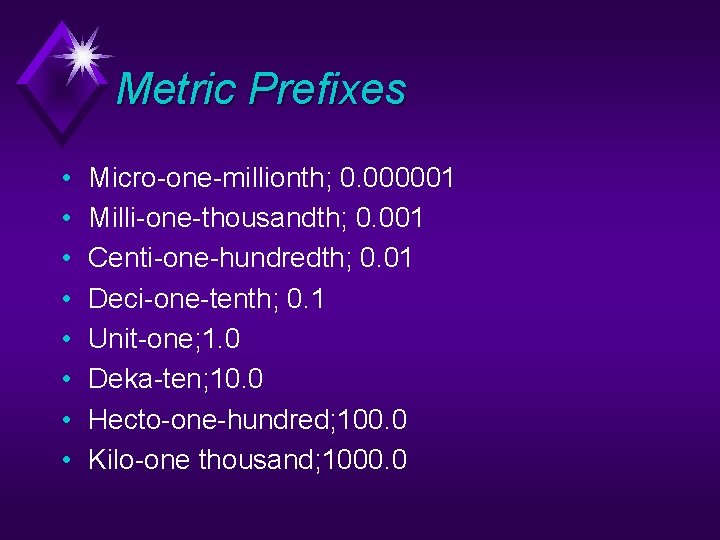
Metric Prefixes • • Micro-one-millionth; 0. 000001 Milli-one-thousandth; 0. 001 Centi-one-hundredth; 0. 01 Deci-one-tenth; 0. 1 Unit-one; 1. 0 Deka-ten; 10. 0 Hecto-one-hundred; 100. 0 Kilo-one thousand; 1000. 0

Metric Equivalents • • Weight (gram) 1 gram=1000 milligrams 1 kilogram=1000 grams 1 ounce=30 grams Capacity (liter) 1 liter=1000 milliliters 1000 liters=1 kiloliter 1 liter=1. 06 quarts • 1 quart=0. 946 liters • 1 milliliter weighs 1 gram • 1 cubic centimeter (cc) and 1 milliliter (ml) are equal/same • 1 liter=1000 ml or cc • 1 fl. oz. =30 ml or cc • 1 fl. dram=0. 125 fl. oz. or 4 ml or cc • 1 gallon=3. 8 liters

Metric Equivalents • • • Length (meter) 1 meter=1000 mm=100 cm=1. 094 yd 1 yard=3 ft. =36 in. =0. 9144 meters 1 in. =2. 54 cm=25. 4 mm 1 micron (micrometer)=0. 001 mm=0. 000001 meter

Metric Abbreviations • • • Gram-gm Milligram-mg Kilogram-kg Liter-L Milliliter-ml • • Cubic centimeter-cc Meter-M Millimeter-mm Centimeter-cm

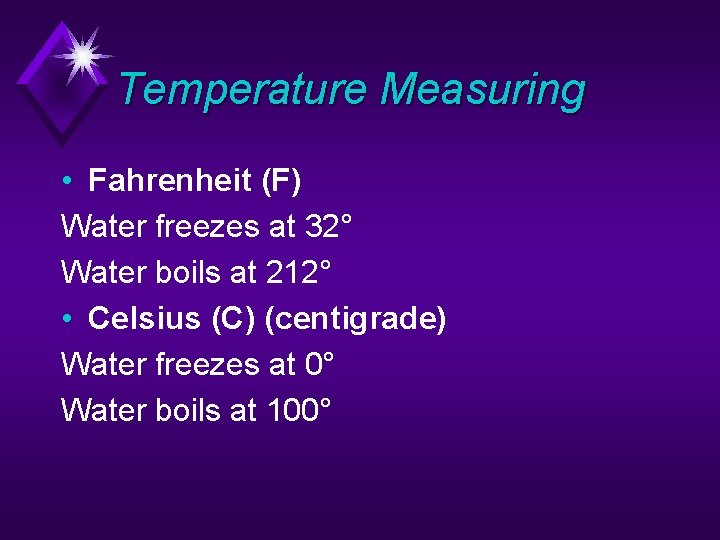
Temperature Measuring • Fahrenheit (F) Water freezes at 32° Water boils at 212° • Celsius (C) (centigrade) Water freezes at 0° Water boils at 100°

Converting C° to F° or F° to C° • • Fahrenheit to Celsius 5/9 (F°-32) = C° ( or F -32 x 5 divided by 9 = C) Celsius to Fahrenheit 9/5 (C°)+32 = F° (or C x 9 divided by 5 + 32 =‘s F)

Dosage Calculations • Formula: • D (desired dose) x Q (quantity of dose on hand) H (dose on hand) =‘s X X = Amount to give/needed • Example: Give 80 mg of a drug from a 30 ml stock bottle labeled “ 100 mg per 1 ml

• I know: 1 ml=100 mg Need to give 80 mg • I do not know: Fraction of ml that=80 mg • Common sense: If 1 ml of the solution = 100 mg of the drug, then the amount given must be less than 1 ml.

• Formula: D x Q = amt to give H • Solution: 80 mg x 1 ml=0. 8 ml of sol. 100 mg • Proof: 80÷ 100=0. 8 0. 8 x 1=0. 8

Standard Dilution Equations • • C 1 x V 1 = C 2 x V 2 C = concentration in % V = volume Problem: Dr. needs 60 ml of ½% contrast media. How much NACL and how much media do you need to make the required amount when you have 60 ml’s of 1% media?

• • First we must convert the % to decimals 1/2% = 0. 5 1 divided by 2 = 0. 5 You always do this when converting percentages to decimals

• C 1=1/2%=0. 5 Concentration 1 (asked for) • C 2=1% = Concentration 2 (given) • V 1=60 ml Volume 1 (asked for) • V 2=x (? volume of saline)

• Formula: C 1 x V 1 = C 2 x V 2 • 0. 5 x 60 =1 x x • 30 = x • So, You add x=30 ml’s of pure saline • Need 60 mlof 1% – 30 ml’s = 30 ml’s of 1% dye solution and add 30 ml’s saline to get 60 ml of 0. 5% dye solution

No Total Amount Asked For? • Procedure calls for 1/2% contrast media. I have 60 ml’s of 1% contrast media. How much NACL do I need to add to the mix to get a ½% solution? • 60 ml’s of 1% contrast media needs to have 60 ml’s of NACL added to the mix to dilute the mixture to ½%. We will be left with 120 ml’s of 1/2 % solution. • The difference is this question and the one above is that the one above calls for 60 ml's as a final amount on your field. Therefore we need 30 ml’s of contrast media and 30 ml’s of NACL to equal 60 ml’s for that question. This question does not specify the ending total amount.

Standard Dilution Equation • Doctor wants ½% lidocaine. You have 25 ml of 1% Lidocaine. How do you dilute it to the proper strength? • C 1 (asked) x V 1 (given) = C 2 (given) x V 2 (? ) • ½% x 25 = 1% x x • 12. 5= x • 25 ml lidocaine – 12. 5 mlof 1% lidocaine = 12. 5 ml saline = ½% or 0. 5% lidocaine

Calculating mg Dose per kg Body Weight • Normal dose of propofol (an induction drug used by anesthesia) is 2 mg per kg for an adult • Find initial dose for an adult weighing 150 lbs • Convert lbs. to kg (2. 2 lbs. = 1 kg) • 150 lbs. ÷ 2. 2 lbs. = 68 kg • 68 kg x 2 mg = 136 mg

Calculating Child Dosages • Clark’s Rule: Standard adult weight=150 lbs • Problem: If an adult receives 75 mg of Demerol, what would be an appropriate dose for a child weighing 30 lbs? • Adult dose=75 mg • Child weighs 30 lbs. • Clark’s rule Standard adult weight = 150 lbs. • Common sense: child is smaller, dose will be less than 75 mg

• Formula: Child’s weight x Adult dose = Child’s dose Adult weight • Solution: • 30 lbs. x 75 mg = 15 mg of Demerol 150 lbs

• The order calls for a dosage of 20 mg’s of a medication that comes in a concentrate of 4 mg’s per ml. How many ml’s do I need? • 20 mg x 4 mg ______ =’s cross multiply. 20 mg x 1 ml = 4 mg x’s x ml • X ml 1 ml • 4 x = 20 divide both sides by 4 • X = 5 mls

• • • What % of NACL is preset in a 2: 3 mixture of tincture and NACL? Hint: There are 5 total parts of the mixture. Total parts =’s 5 So 2/5’s is tincture = ( 2 divided by 5 = 0. 4) 40% 3/5’s is NACL = ( 3 divided by 5 = 0. 6) 60% #41. You have a 4: 5: 1 solution of water, alcohol, and tincture. What % of the solution does each ingredient represent? 4 = water or 4/10 or 40% 5 =’s alcohol or 5/10’s or 50% 1 =’s tincture or 1/10 or 10%

Mixing Medications • Do not mix drugs unless you know for certain that they are compatible together • If a compatibility is questioned direct the question to the pharmacist to be certain before mixing • Mixing drugs that are incompatible can result in decreased or increased efficacy, precipitates/crystallization of the drugs which could cause embolization of the drug in the patient, or death • Institutional pharmacies keep a compatibility chart in the pharmacy/Some ORs may have them in the room

Summary • • • Pharmacokinetics Pharmacodynamics Actions/Effects Abbreviations Nomenclature Legal implications • Metric System • Conversions/ Mathematics • Dosage Calculations • Mixing Medications
 Pharmacology of drugs acting on respiratory system
Pharmacology of drugs acting on respiratory system Adrenal drugs pharmacology
Adrenal drugs pharmacology Venipuncture for radiologic technologists
Venipuncture for radiologic technologists Chapter 15 diagnostic procedures and pharmacology
Chapter 15 diagnostic procedures and pharmacology Toxicology and applied pharmacology
Toxicology and applied pharmacology Annual review of pharmacology and toxicology
Annual review of pharmacology and toxicology In fair verona, where we lay our scene modern translation
In fair verona, where we lay our scene modern translation Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Giọng cùng tên là
Giọng cùng tên là Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Bảng số nguyên tố
Bảng số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ